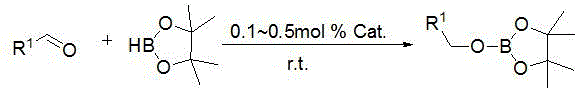Application of trisilazane-rare earth complex in catalysis of hydroboration reaction between aldehyde and borane
A technology of rare earth complexes and trisilicon amines, applied in catalytic reactions, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve the problems of long reaction time, low substrate universality, The problem of large amount of catalyst is used to achieve the effect of fast reaction speed, simple and controllable reaction process, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
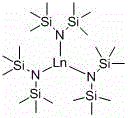
[0025] Embodiment one: La[N(SiMe 3 ) 2 ] 3 Catalytic synthesis of boronate from benzaldehyde and pinacol borane
[0026] Under an inert gas atmosphere, the catalyst La[N(SiMe 3 ) 2 ] 3 hexane solution (0.1 mL, 0.01 mol / L), then add pinacol borane (0.145 mL, 1 mmol) with a pipette, then add benzaldehyde (0.101 mL, 1 mmol) with a pipette, room temperature After reacting for 10min, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 95%. Dry CDCl under reduced pressure 3 and a mixed solution of hexane, add n-hexane (3 × 2mL), and drain to obtain the corresponding pinacol borate, C 6 h 5 CH 2 OB(OC(CH 3 ) 2 C(CH 3 ) 2 O).
Embodiment 2
[0027] Embodiment two: La[N(SiMe 3 ) 2 ] 3 Catalytic synthesis of boronate from benzaldehyde and pinacol borane
[0028] Under an inert gas atmosphere, the catalyst La[N(SiMe 3 ) 2 ] 3 hexane solution (0.25 mL, 0.01 mol / L), then add pinacol borane (0.145 mL, 1 mmol) with a pipette, and then add benzaldehyde (0.101 mL, 1 mmol) with a pipette, room temperature After reacting for 10 min, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 94%. Dry CDCl under reduced pressure 3 and a mixed solution of hexane, add n-hexane (3 × 2mL), and drain to obtain the corresponding pinacol borate, C 6 h 5 CH 2 OB(OC(CH 3 ) 2 C(CH 3 ) 2 O).
Embodiment 3
[0029] Embodiment three: La[N(SiMe 3 ) 2 ] 3 Catalytic synthesis of boronate from benzaldehyde and pinacol borane
[0030] Under an inert gas atmosphere, the catalyst La[N(SiMe 3 ) 2 ] 3 hexane solution (0.5 mL, 0.01 mol / L), then add pinacol borane (0.145 mL, 1 mmol) with a pipette, then add benzaldehyde (0.101 mL, 1 mmol) with a pipette, room temperature After reacting for 10 min, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 90%. Dry CDCl under reduced pressure 3 and a mixed solution of hexane, add n-hexane (3 × 2mL), and drain to obtain the corresponding pinacol borate, C 6 h 5 CH 2 OB(OC(CH 3 ) 2 C(CH 3 ) 2 O).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com