A kind of ciprofloxacin hydrochloride sustained-release microcapsules for animals and preparation method thereof
A technology for ciprofloxacin hydrochloride and animals, which is applied in the direction of microcapsules, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of reducing animal morbidity, unsatisfactory conditions, and affecting treatment effects, etc. problems, to achieve the effects of prolonging the average residence time, good palatability, and good sustained-release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of preparation method of animal-specific ciprofloxacin hydrochloride sustained-release microcapsules
[0036] (1) 90kg of stearic acid is melted in a 70°C water bath, 15kg of ciprofloxacin hydrochloride is added, stirred and evenly mixed, the molten material is spread out to condense, solidify, pulverize, and sieve to obtain the core material;
[0037] (2) Dissolve 4.0kg Eudragit E 100 (copolymer of butyl methacrylate, dimethylaminoethyl methacrylate and methyl methacrylate (1:2:1)) in 200L purified water to obtain 2% Eudragit E 100 coating liquid;
[0038] (3) The core material and the coating solution were mixed, and then spray-dried. The spray-drying inlet air temperature was 110° C., the outlet air temperature was 55° C., and the flow rate was 5 mL / min to obtain microencapsulated ciprofloxacin hydrochloride.
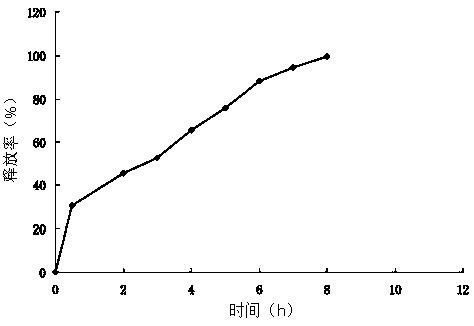
[0039] The obtained ciprofloxacin hydrochloride sustained-release microcapsules have stable physical and chemical properties, and the drug loading and...
Embodiment 2
[0042] A kind of preparation method of animal-specific ciprofloxacin hydrochloride sustained-release microcapsules
[0043] (1) Melting 90kg of stearic acid in a 70°C water bath, adding 10kg of ciprofloxacin hydrochloride, stirring and mixing, the molten material is spread out to condense, solidify, pulverize, and sieve to obtain the core material;
[0044] (2) Dissolve 4.0kg Eudragit E 100 (copolymer of butyl methacrylate, dimethylaminoethyl methacrylate and methyl methacrylate (1:2:1)) in 200L purified water to obtain 2% Eudragit E 100 coating solution;
[0045] (3) The core material and the coating solution were mixed, and then spray-dried. The spray-drying inlet air temperature was 110° C., the outlet air temperature was 55° C., and the flow rate was 5 mL / min to obtain microencapsulated ciprofloxacin hydrochloride.
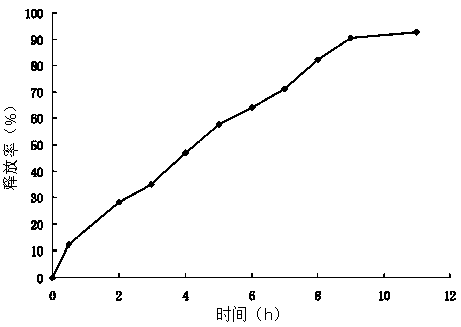
[0046] The obtained ciprofloxacin hydrochloride sustained-release microcapsules have stable physical and chemical properties, and the drug loading and encaps...
Embodiment 3
[0049] A kind of preparation method of animal-specific ciprofloxacin hydrochloride sustained-release microcapsules
[0050] (1) 90kg of stearic acid is melted in a 70°C water bath, 18kg of ciprofloxacin hydrochloride is added, stirred and evenly mixed, the melted material is spread out to condense, solidify, pulverize, and sieved to obtain a core material;
[0051] (2) Dissolve 4kg Eudragit E 100 (copolymer of butyl methacrylate, dimethylaminoethyl methacrylate and methyl methacrylate (1:2:1)) in 200L purified water to obtain 2 % Eudragit E 100 coating solution;
[0052] (3) The core material and the coating solution were mixed, and then spray-dried. The spray-drying inlet air temperature was 110° C., the outlet air temperature was 55° C., and the flow rate was 5 mL / min to obtain microencapsulated ciprofloxacin hydrochloride.
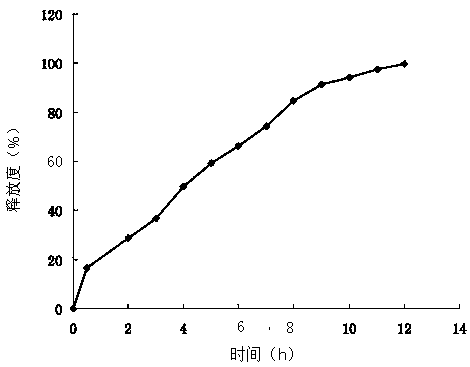
[0053] The obtained ciprofloxacin hydrochloride sustained-release microcapsules have stable physical and chemical properties, and the drug loading and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com