Application of chelerythrine to treatment of poultry drug-resisting colibacillosis
A technology of chelerythrine and colibacillosis, applied to medical preparations containing active ingredients, antibacterial drugs, pharmaceutical formulas, etc., to achieve good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
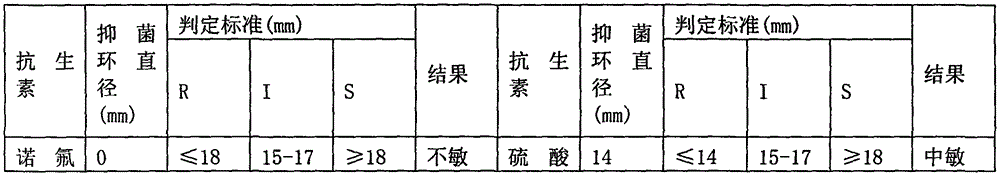
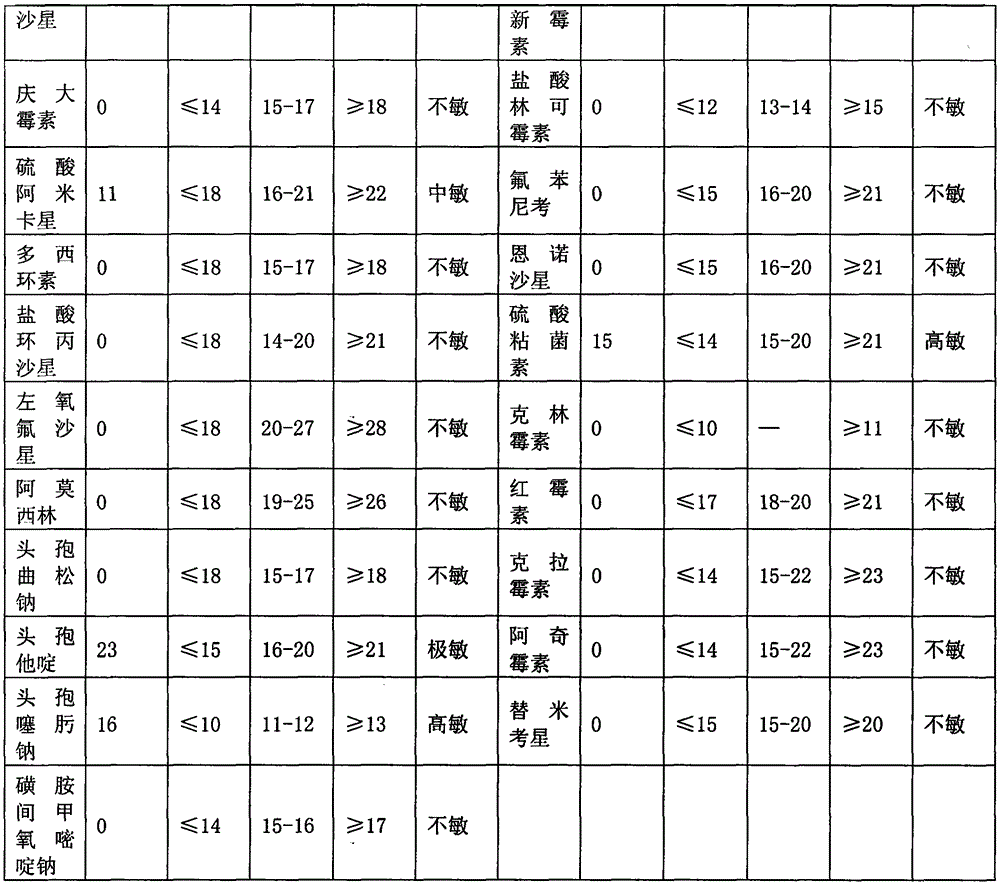
Image
Examples
specific Embodiment 1
[0016] The extraction method of specific embodiment 1 chelerythrine
[0017] Extraction method of chelerythrine in blood water grass. Extraction: Weigh an appropriate amount of sample, add hydrochloric acid aqueous solution with a pH value of 1, put it in a water bath, and extract for 30 minutes at 100°C to extract sanguinarine and chelerythrine. Separation: Utilize macroporous adsorption resin to adsorb and separate sanguinarine and chelerythrine in blood water grass, and use 100% ethanol to elute and separate. The method has the advantages of less pollution, economy, good repeatability, high product yield and high purity, and is applicable to industrial production.
[0018] The extraction method of celandine in celandine: take 2 g of celandine powder, add 70% ethanol solution with a solid-liquid ratio of 1:20, extract for 30 minutes under the condition of 700w ultrasonic power, and use macroporous adsorption resin to absorb and separate . The extraction rate of chelerythr...
specific Embodiment 2
[0019] Specific Example 2 The assay method of chelerythrine
[0020] Determined by high performance liquid chromatography.
[0021] Chromatographic conditions and system adaptability test: using octadecylsilane bonded silica gel as filler; acetonitrile-0.1% phosphoric acid solution (25:75) as mobile phase; detection wavelength is 282nm; flow rate 1.0ml / min; theoretical column The number of plates shall not be less than 3000 based on the chelerythrine peak.
[0022] Preparation of reference substance solution: Take 10 mg of chelerythrine reference substance, accurately weigh it, put it in a 100ml measuring bottle, add methanol to dissolve and dilute to the mark, accurately measure 5ml, put it in a 10ml measuring bottle, add methanol to the mark, Shake well, filter, and take the continued filtrate, that is.
[0023] Preparation of the test solution: Take 10 mg of this product, accurately weigh it, put it in a 100ml measuring bottle, add methanol to dissolve and dilute to the m...
specific Embodiment 3
[0026] Specific embodiment 3 preparation preparation method
[0027] 1. Preparation of granules:
[0028] Take 1 part of the extracted culerythrine, 3 parts of powdered sugar, and 1 part of dextrin, pass through a 80-mesh sieve, mix evenly, use 80% alcohol to make a suitable soft material, and pass the soft material through a 14-mesh sieve to granulate , dried at 60°C, and the dried granules were sieved with a 12-mesh sieve and packaged.
[0029] 2. Preparation of injections:
[0030] Injection: Take 20 grams of air-dried or sun-dried whole grass powder, add PH4 sulfuric acid aqueous solution, decoct twice, 30 minutes each time. Filter through gauze, combine the filtrates, and concentrate to a thick syrup. Slowly add sodium hydroxide solution to PH9-10, let it stand, filter out the brown-black precipitate precipitated in the solution, add ethanol to dissolve it, filter with No. 3 vertical melting funnel, add ethanol to the filtrate, and then add PH4-5 phosphoric acid aqueou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com