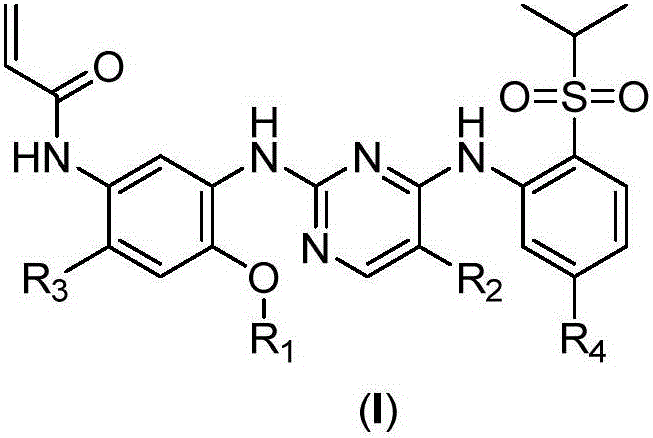
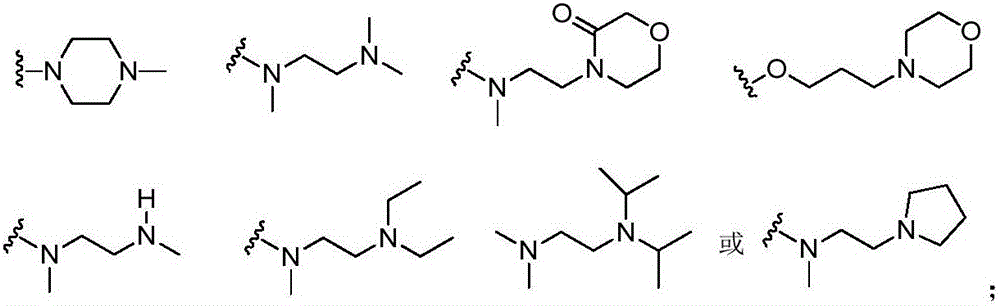
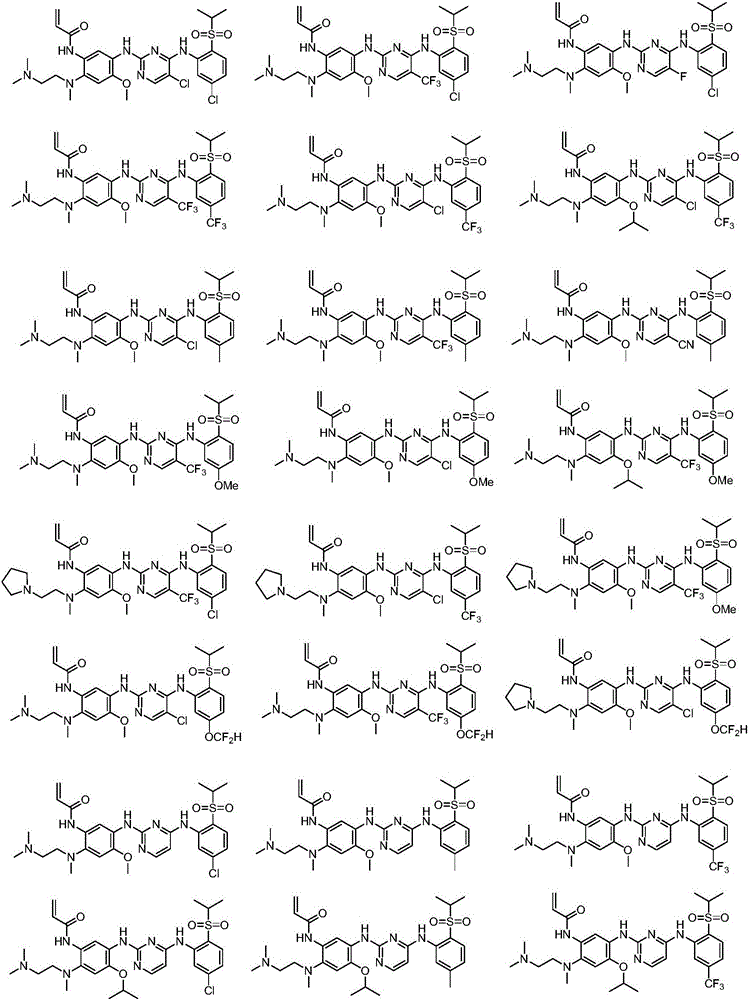
Inhibitors with ALK and EGFR dual activity, and preparation method and application thereof
A phenyl, amino technology, applied in the field of drug synthesis, can solve the problems of limited dosage, poor selectivity, tumor recurrence, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: N-(5-((4-((5-chloro-2-(isopropylsulfonyl)phenyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino) - Preparation of 2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxyphenyl)acrylamide
[0070]
[0071] The first step: the preparation of 4-chloro-2-(isopropylthio)aniline
[0072]
[0073] Put 5-chloro-2-aminothiophenol (500mg, 3.13mmol), potassium carbonate (973mg, 7.04mmol) in a 50mL round bottom bottle, add 15ml DMF, add isopropyl bromide (0.5mL) under stirring , the mixture was stirred overnight at room temperature, 50 ml of water was added, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, and the solvent was distilled off. The residue was subjected to silica gel column chromatography to obtain a light yellow oily viscous liquid (0.520 g).
[0074] The second step: the preparation of 4-chloro-2-(isopropylsulfonyl)aniline
[0075]
[0076] 4-Chloro-2-(isopropylthio)aniline (520mg, 3.33mmol) and m-chloroperoxybenzoic acid ...
Embodiment 2
[0099] Example 2: N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-5-((4-((2-(isopropylsulfonyl)-5-(tri Preparation of fluoromethyl)phenyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-4-methoxyphenyl)acrylamide
[0100]
[0101] The first step: preparation of isopropyl (2-nitro-4-(trifluoromethyl)phenyl)sulfane
[0102]
[0103] Take a 100mL single-necked bottle, add 1-fluoro-2-nitro-4-(trifluoromethyl)benzene (1g) and 15mL DMF, add potassium carbonate (3.3g) and isopropylthiol (0.72g) to the above solution respectively , stirred at 50°C for 3 hours, cooled to 0°C, added 2ml of NaOH (1M) aqueous solution, extracted with ethyl acetate, dried, and concentrated to give a white solid (2.5g).
[0104] 1 H NMR (400MHz, CDCl3) δ8.44(s, 1H), 7.76(d, J=8.5Hz, 1H), 7.76(d, J=8.5Hz, 1H), 7.58(d, J=8.5Hz, 1H ),7.58(d,J=8.5Hz,1H),3.62(dt,J=13.3,6.7Hz,1H),3.62(dt,J=13.3,6.7Hz,1H),1.44(d,J=6.7Hz ,6H).
[0105] The second step: the preparation of 1-(isopropylsulfonyl)-2-nitro-4-(tri...
Embodiment 3
[0128] Example 3: N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-5-((4-((2-(isopropylsulfonyl)-5-methyl Preparation of phenyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-4-methoxyphenyl)acrylamide
[0129]
[0130] The first step: the preparation of 1-(isopropylsulfonyl)-4-methyl-2-nitrobenzene
[0131]
[0132] 2-Fluoro-5-methylnitrobenzene (3.0 g, 19.355 mmol) was dissolved in DMF (50 mL), and isopropylthiol (2.95 g, 38.71 mmol) and potassium carbonate (8.01 g, 58.065 mmol) were added. The reaction was stirred at 60°C for 2 hours, and LCMS showed that the reaction was complete. The reaction solution was dissolved in dichloromethane, washed with water and saturated sodium chloride, the organic phase was dried and filtered, concentrated, the residue was dissolved in DCM (100 mL), and m-chloroperoxybenzoic acid (8.35 g, 48.387 mmol) was added in portions. Stir for 16 hours, LCMS shows that the reaction is complete, the reaction solution is quenched with saturated sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com