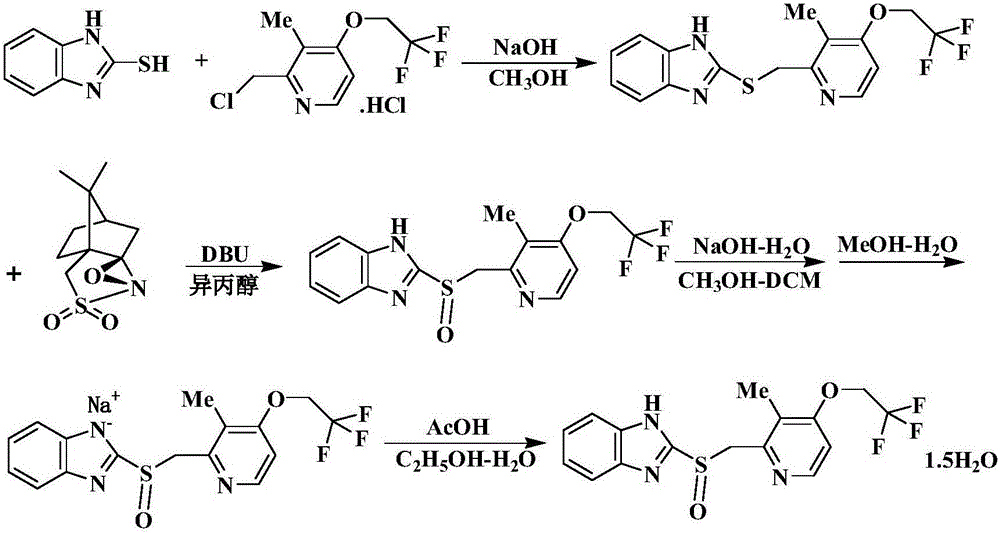
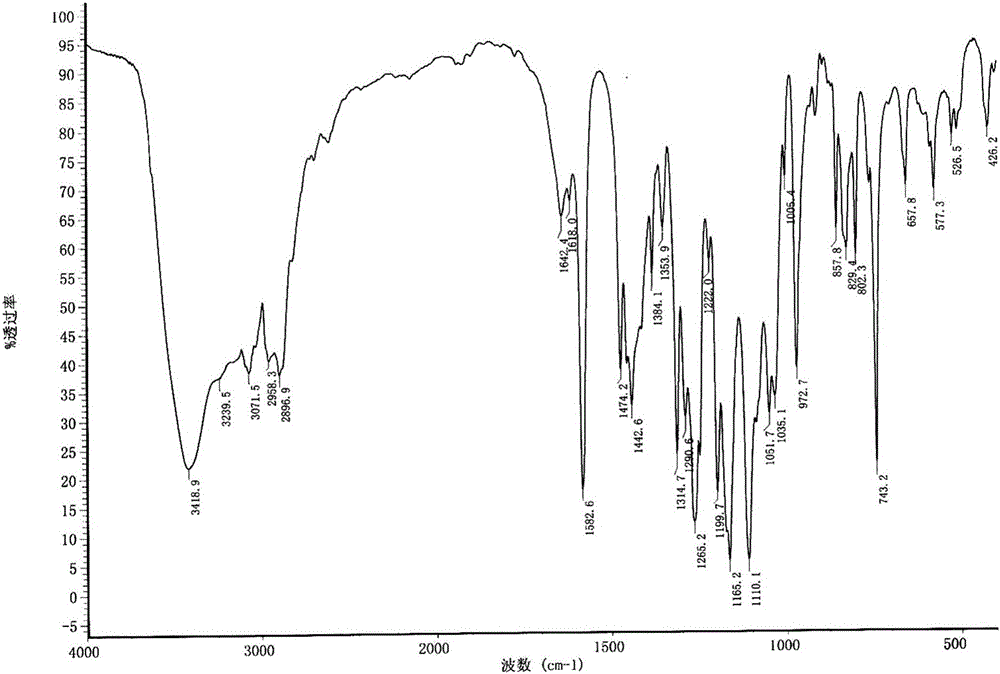
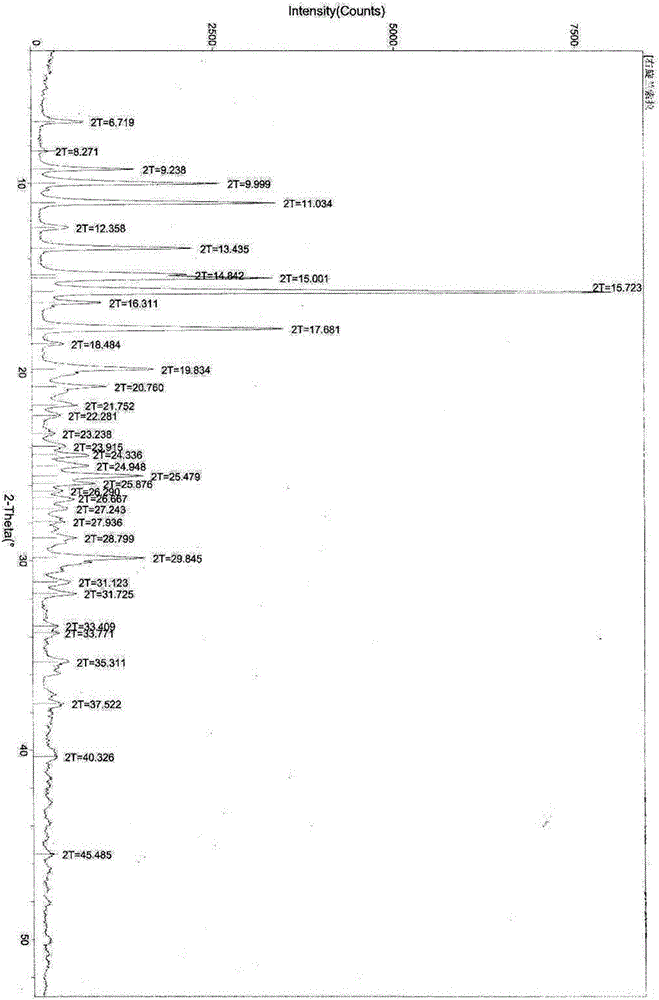
Synthesis method of R-lansoprazole
A technology of dexlansoprazole and dexlansoprazole sodium is applied in the field of drug synthesis, which can solve the problems of many types of raw materials, cumbersome operations, and low prices, and achieve convenient post-processing, high conversion rate of raw materials, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Step 1: Preparation of lansoprazole sulfide.
[0054] Material ratio
[0055] Step 1 material ratio table
[0056]
[0057] Operation process:
[0058] ①.In a 100L enamel reaction kettle, add 30kg of anhydrous methanol, then add 5kg of 3-methyl-4-(2,2,2-trifluoroethoxy)-2-chloromethylpyridine hydrochloride, 2 -Mercaptobenzimidazole 3kg, stirred for 30min.
[0059] ②. Slowly add 2.54kg of sodium hydroxide in 5 times, wash the kettle wall with the remaining 2kg of methanol, and react at 70±5°C for 3 hours.
[0060] Reaction monitoring: TLC monitoring [TLC material: G 254 , thickness: 0.2-0.25mm; developing agent, ethyl acetate: petroleum ether (4:3), 254 / 345nm ultraviolet color development] until the conversion of raw materials is complete.
[0061] ③. After the reaction is completed, lower the temperature to about 40°C, and recover methanol (about 35L) under reduced pressure at 40-45°C. The residue was a light yellow oily substance, 50 kg of purified water (the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com