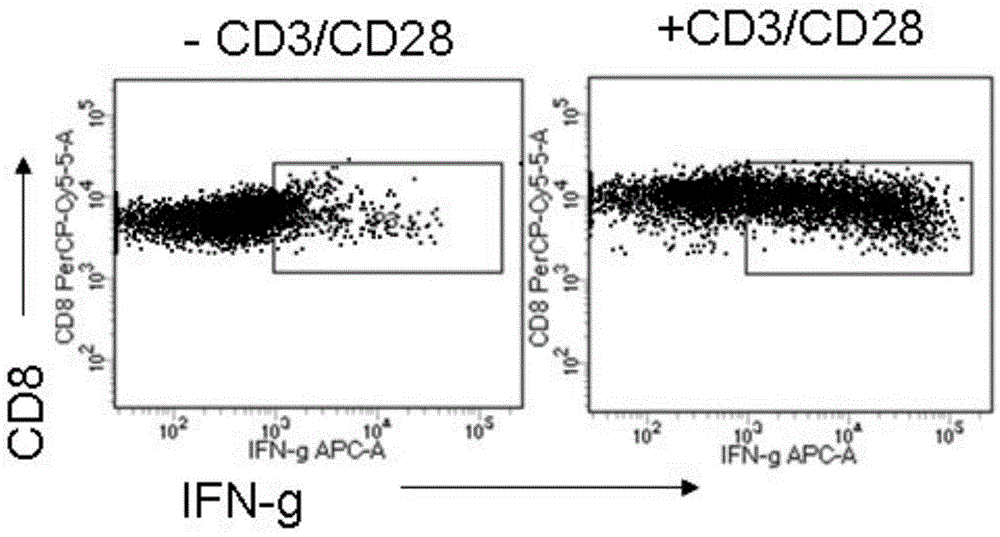
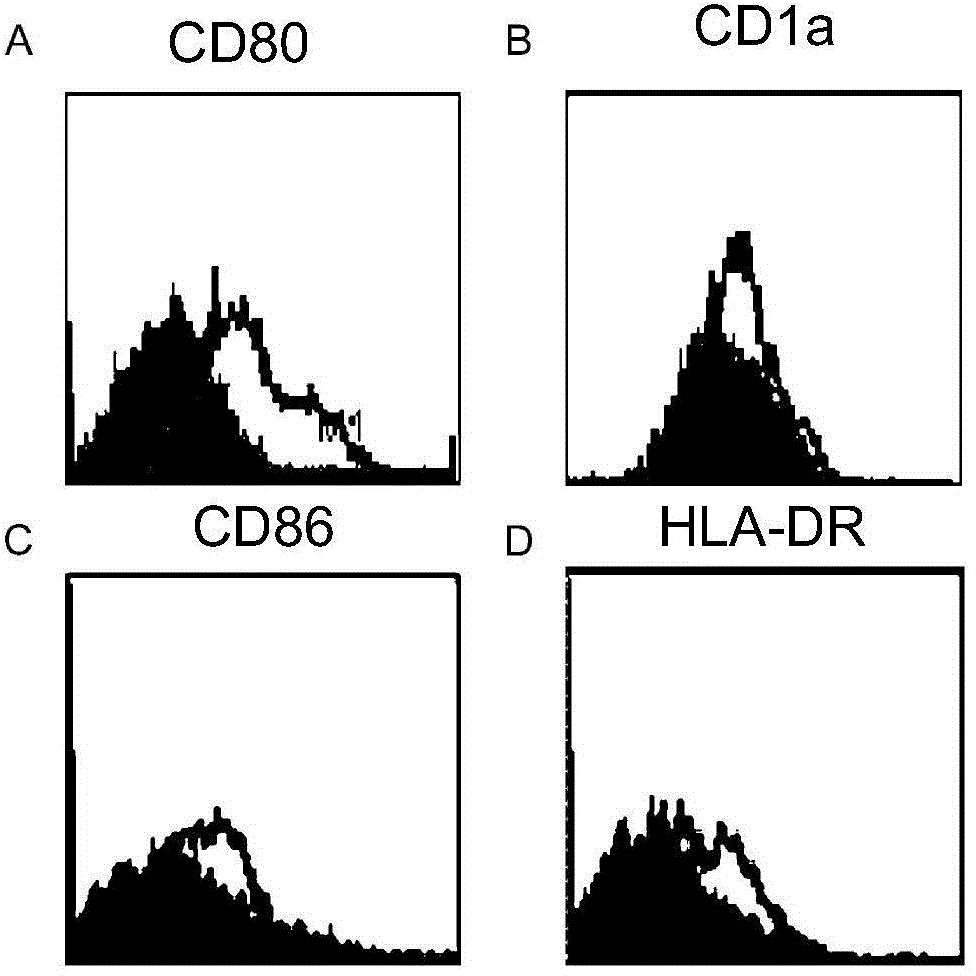
Serum-free culture medium for adoptive immune cells
A technology of serum-free medium and immune cells, applied in the field of cell engineering, to achieve stable product performance, promote the formation of lentivirus-infected primary T lymphocytes and carT cells, and have strong reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] T cell culture
[0022] The formulation of the serum-free medium for adoptive immune cells provided by this embodiment is as follows:
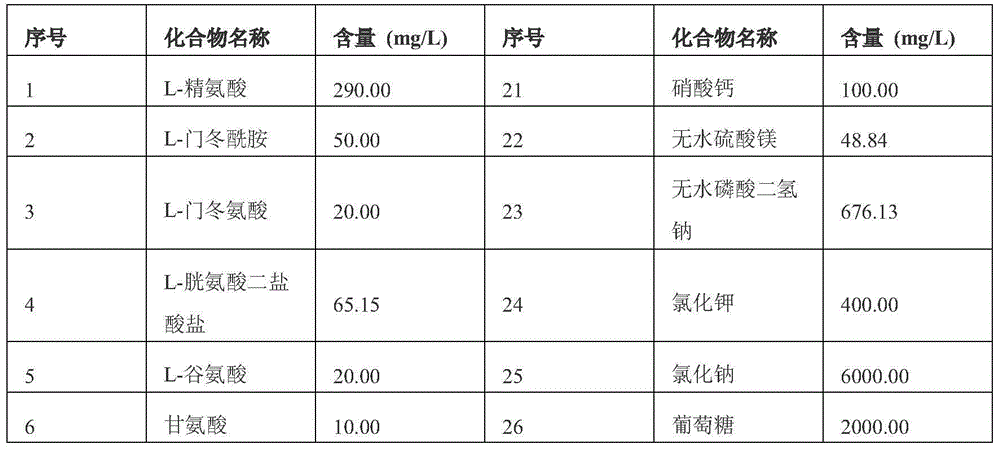
[0023] RPMI 1640 medium, and additional nutritional components added to the RPMI 1640 medium, the types of nutritional components and the added quality per unit volume are as follows: L-arginine 156mg / L, L-asparagine 53mg / L, L-Aspartic Acid 17mg / L, L-Glutamic Acid 19mg / L, L-Glutamine 285mg / L, Glycine 17mg / L, L-Histidine 23.5mg / L, L-Isoleucine 53mg / L, L-polylysine sodium 33mg / L, L-methionine 23.5mg / L, L-phenylalanine 21mg / L, L-proline 19mg / L, glucose 2500mg / L, L-serine 28mg / L, L-threonine 20.5mg / L, L-tryptophan 11mg / L, L-valine 23.5mg / L, L-leucine 53mg / L, β-mercaptoethanol 3.2mg / L , L-tyrosine disodium dihydrate 304mg / L, ethanolamine 2.5mg / L, L-cystine dihydrochloride 53mg / L, dexamethasone 0.002mg / L, CuSO 4 ·5H 2 O0.0009mg / L, MnCl 2 4H 2 O 0.00045mg / L, Ni(NO3) 2 ·6H 2 O 0.00007mg / L, ZnSO 4 ·7H 2 O 0.19mg / L, CoCl 2 ·6H 2 O 0...
Embodiment 2
[0032] T cell culture
[0033] The formulation of the serum-free medium for adoptive immune cells provided by this embodiment is as follows:
[0034] Get the RPMI 1640 culture medium of unit volume, add the nutrient component in the RPMI 1640 culture medium unit volume addition amount as follows: L-arginine 165mg / L, L-asparagine 56mg / L, L-asparagine Acid 18mg / L, L-glutamic acid 20mg / L, L-glutamine 300mg / L, glycine 18mg / L, L-histidine 25mg / L, L-isoleucine 56mg / L, L-poly Sodium polylysine 35mg / L, L-methionine 25mg / L, L-phenylalanine 23mg / L, L-proline 20mg / L, glucose 2500-3000mg / L, L-serine 30mg / L, L - Threonine 22mg / L, L-Tryptophan 12mg / L, L-Valine 25mg / L, L-Leucine 55mg / L, β-Mercaptoethanol 3.5mg / L, L-Tyramine Dihydrate Disodium acid 320mg / L, ethanolamine 3mg / L, L-cystine dihydrochloride 55mg / L, dexamethasone 0.003mg / L, CuSO 4 5H2O 0.001mg / L, MnCl 2 4H 2 O 0.0005mg / L, Ni(NO3) 2 ·6H 2 O 0.00008mg / L, ZnSO 4 ·7H 2 O 0.2mg / L, CoCl 2 ·6H 2 O 0.005mg / L, SnC1 2 2H 2 O0.0...
Embodiment 3
[0043] T cell culture
[0044] RPMI 1640 medium, and additional nutritional components added to the RPMI 1640 medium, the types of nutritional components and the added quality per unit volume are as follows: L-arginine 174mg / L, L-asparagine 59mg / L, L-aspartic acid 19mg / L, L-glutamic acid 21mg / L, L-glutamine 315mg / L, glycine 19mg / L, L-histidine 26.5mg / L, L-iso Leucine 59mg / L, L-polylysine sodium 37mg / L, L-methionine 26.5mg / L, L-phenylalanine 24.5mg / L, L-proline 21mg / L, glucose 3000mg / L L, L-serine 32mg / L, L-threonine 23mg / L, L-tryptophan 13mg / L, L-valine 26.5mg / L, L-leucine 58mg / L, β-mercaptoethanol 3.7mg / L, L-tyrosine disodium dihydrate 336mg / L, ethanolamine 3.2mg / L, L-cystine dihydrochloride 58mg / L, dexamethasone 0.004mg / L, CuSO 4 ·5H 2 O 0.0011mg / L, MnCl 2 4H 2 O 0.00055mg / L, Ni(NO3) 2 ·6H 2 O0.00009mg / L, ZnSO 4 ·7H 2 O 0.21mg / L, CoCl 2 ·6H 2 O 0.006mg / L, SnC1 2 2H 2 O 0.00007 mg / L, Na 2 SeO 3 0.01mg / L,FeSO 4 ·7H 2 O 1.3mg / L, vitamin C 0.55mg / L, p-hydroxyb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com