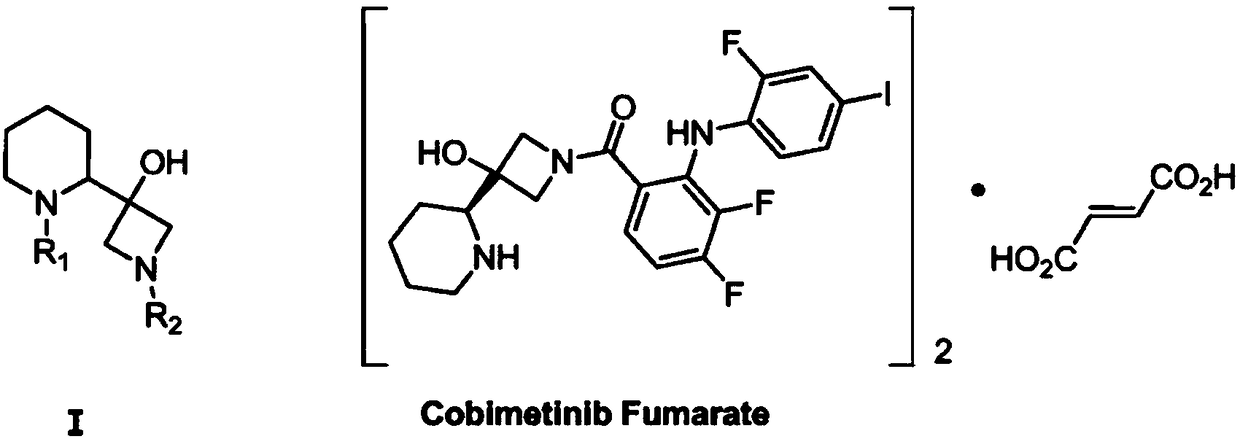
A kind of synthetic method of s-3-(piperidin-2-yl)-azetidin-3-alcohol
A technique for the synthesis of azetidine and its synthesis method, which is applied in the field of synthesis of S-3--azetidine-3-ol, and can solve problems such as difficulty in realizing industrialized mass production, non-compliance with environmental protection requirements, and complex process schemes, etc. problem, to achieve the effect of good methodological significance, easy operation, and short process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
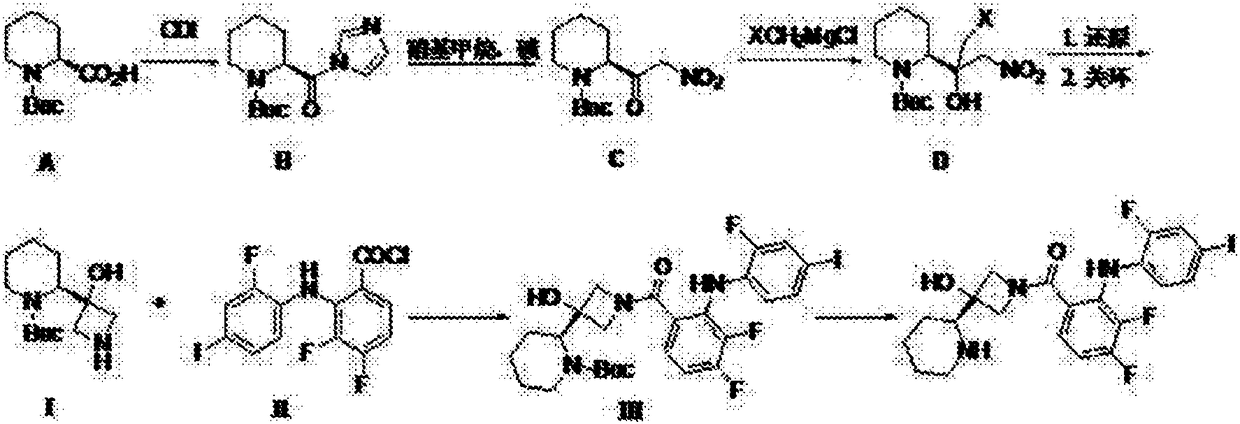
[0050] Embodiment 1: A kind of specific synthetic method of S-3-(piperidin-2-yl)-azetidin-3-alcohol
[0051] S1. The compound of formula A S-N-Boc-piperidine-2-carboxylic acid is condensed with CDI to obtain the compound of formula B; the molar ratio of the compound of formula A S-N-Boc-piperidine-2-carboxylic acid to CDI is 1:1; the reaction system The solvent used is tetrahydrofuran; the temperature of the reaction is 0°C;
[0052] S2. The compound of formula B and nitromethane are condensed under alkaline conditions to obtain the compound of formula C; the molar ratio of the compound of formula B, nitromethane and alkali is 1:1:1; the alkali is sodium alkoxy salt; the solvent used in the reaction system is tetrahydrofuran; the temperature of the reaction is 0°C, and the reaction time is 1h;
[0053] S3. Formula C compound reacts with halomethyl Grignard reagent to obtain formula D compound; The molar ratio of formula C compound and halomethyl Grignard reagent is 1:1; Descr...
Embodiment 2
[0057] Embodiment 2: A kind of specific synthetic method of S-3-(piperidin-2-yl)-azetidin-3-alcohol
[0058] S1. The compound of formula A S-N-Boc-piperidine-2-carboxylic acid is condensed with CDI to obtain the compound of formula B; the molar ratio of the compound of formula A S-N-Boc-piperidine-2-carboxylic acid to CDI is 1:2; the reaction system The solvent used is DMF; the temperature of the reaction is 60°C;
[0059] S2. The compound of formula B and nitromethane are condensed under alkaline conditions to obtain the compound of formula C; the molar ratio of the compound of formula B, nitromethane and alkali is 1:2:2; the alkali is potassium of alkoxy salt; the solvent used in the reaction system is dioxane; the temperature of the reaction is 60°C, and the reaction time is 48h;
[0060] S3. Formula C compound reacts with halomethyl Grignard reagent to obtain formula D compound; The molar ratio of formula C compound and halomethyl Grignard reagent is 1:2; Described halome...
Embodiment 3
[0064] Embodiment 3: A kind of specific synthetic method of S-3-(piperidin-2-yl)-azetidin-3-alcohol
[0065] S1. The compound of formula A S-N-Boc-piperidine-2-carboxylic acid is condensed with CDI to obtain the compound of formula B; the molar ratio of the compound of formula A S-N-Boc-piperidine-2-carboxylic acid to CDI is 1:1.2; the reaction system The solvent used is DMAc; the temperature of the reaction is 8°C;
[0066] S2. The compound of formula B and nitromethane are condensed under alkaline conditions to obtain the compound of formula C; the molar ratio of the compound of formula B, nitromethane and alkali is 1:1.1:1.2; the alkali is an alkali metal hydride; The solvent used in the reaction system is DMF; the reaction temperature is 10°C, and the reaction time is 5h;
[0067] S3. The compound of formula C reacts with the halomethyl Grignard reagent to obtain the formula D compound; the molar ratio of the formula C compound to the halomethyl Grignard reagent is 1:1.2;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com