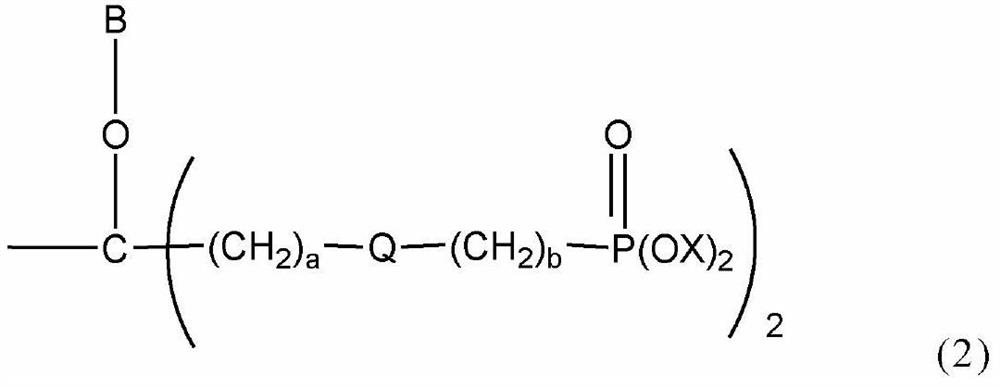
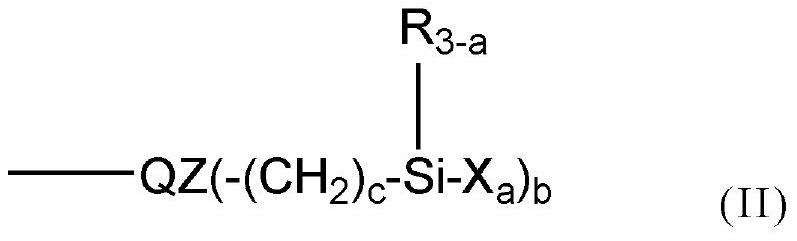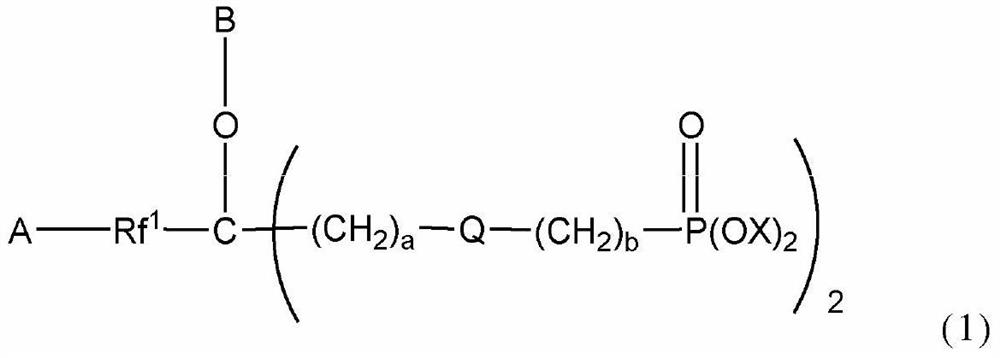Polymer-modified phosphonic acid derivative containing fluoroalkylene oxide and surface treatment agent containing same
A technology of oxyalkylene and phosphonic acid derivatives, used in biocide-containing paints, polyether coatings, other chemical processes, etc., to achieve excellent low dynamic friction, excellent detergency, and excellent adhesion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] craft (1i)
[0118] In a reaction vessel, 150 g of tetrahydrofuran and 300 g of 1,3-bistrifluoromethylbenzene were mixed, and 160 ml of 0.7 M allylmagnesium bromide was added dropwise. Next, after slowly adding 300 g of the compound represented by the following formula (1a) dropwise, it heated at 60 degreeC for 4 hours.
[0119]
[0120] After the heating was completed, it was cooled to room temperature, and the solution was added dropwise to 300 g of 1.2 M hydrochloric acid aqueous solution to stop the reaction. After recovering the lower fluorine compound layer by a liquid separation operation, it was washed with acetone. The fluorine compound in the lower layer is recovered after cleaning. The solvent and unreacted substances were distilled off to obtain 290 g of a compound represented by the following formula (1b).
[0121]
[0122] Craft (1ii)
[0123] Next, 20 g of the compound obtained in the above process (1i) (formula (1b)), 30 g of 1,3-trifluoromethy...
Embodiment 2
[0139] 20g of the compound (formula (1d)) obtained in Example 1 and 30g of 1,3-trifluoromethylbenzene and 10g of diethyl ether and 3.250g of bromotrimethylsilane were mixed at 70 Cake for 24 hours. Then, the solvent and unreacted substances were distilled off under reduced pressure to obtain 20 g of a liquid product. pass 1 The obtained mixture was measured by H-NMR, and it was confirmed that it was represented by following formula (2e).
[0140]
[0141] In formula (2e), X is CH 2 CH 3 or Si(CH 3 ) 3 .
[0142] CH 2 CH 3 : Si(CH 3 ) 3 =59:41
[0143] (p / q=0.9, )
[0144] The compound of the above formula (2e) (hereinafter referred to as 'compound 3') 1 The data of H-NMR (TMS standard, ppm) are shown below.
[0145]
Embodiment 3
[0147] Craft (3i)
[0148] 20 g of the compound (formula (1b)) obtained in Example 1, 30 g of 1,3-trifluoromethylbenzene and 30 g of 1,4-bis(dimethylsilyl)benzene and 0.005 g of Toluene solution of chloroplatinic acid / vinylsiloxane complex (containing 1.25×10 -9 mol) were mixed and aged at 80°C for 5 hours. Then, the solvent and unreacted substances were distilled off under reduced pressure to obtain 21 g of a liquid product. pass 1 The obtained compound was measured by H-NMR, and it was confirmed that it was represented by the following formula (3c).
[0149]
[0150] Craft (3ii)
[0151] Next, 20 g of the compound (formula (3c)) obtained in the above process (3i) and 30 g of 1,3-trifluoromethylbenzene and 4.0 g of allylphosphonic acid diethyl ester and 0.005 g of Toluene solution of chloroplatinic acid / vinylsiloxane complex (containing 1.25×10 -9 mol) were mixed and aged at 90°C for 48 hours. Then, the solvent and unreacted substances were distilled off under reduc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com