Framework material for adsorption and separation of CO2 and preparation method of framework material
A framework material, carbon dioxide technology, applied in separation methods, chemical instruments and methods, dispersed particle separation, etc., can solve the problems of poor selectivity of adsorbents, and achieve the effects of high selectivity, good application prospects, and cheap preparation of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
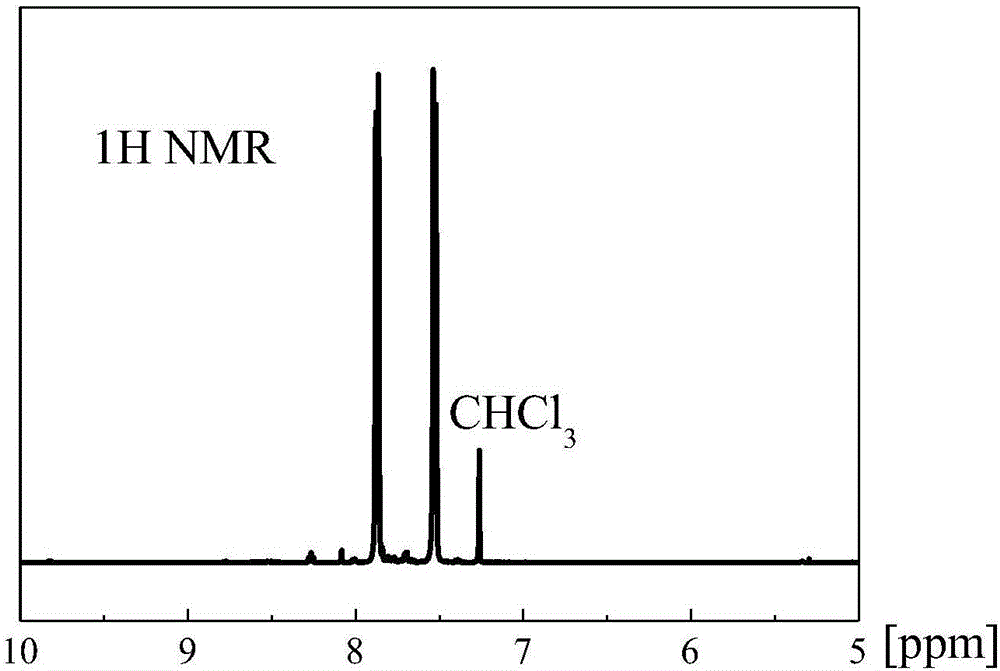
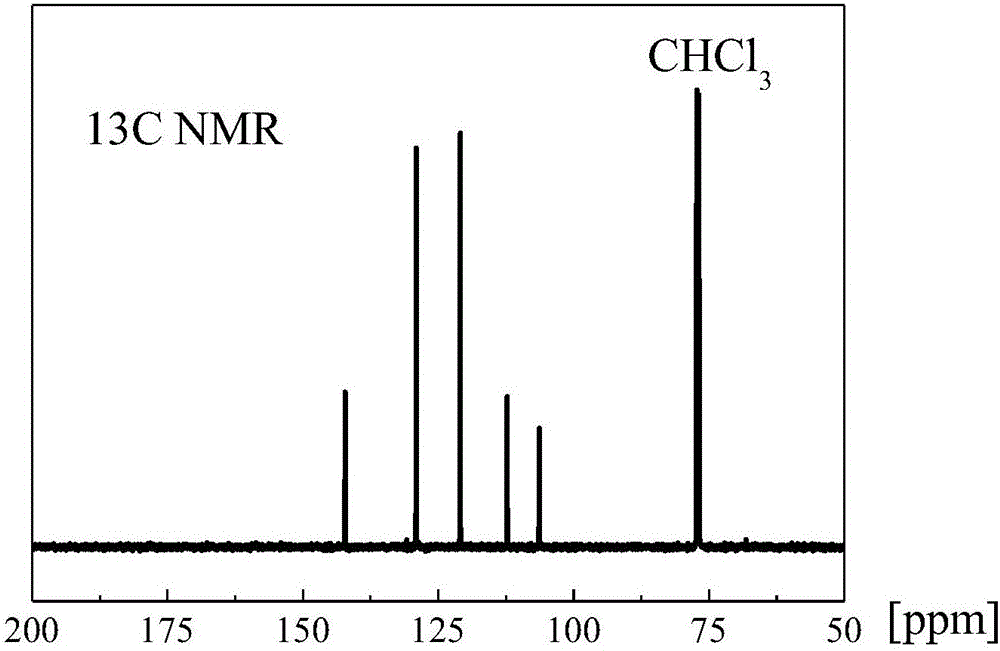
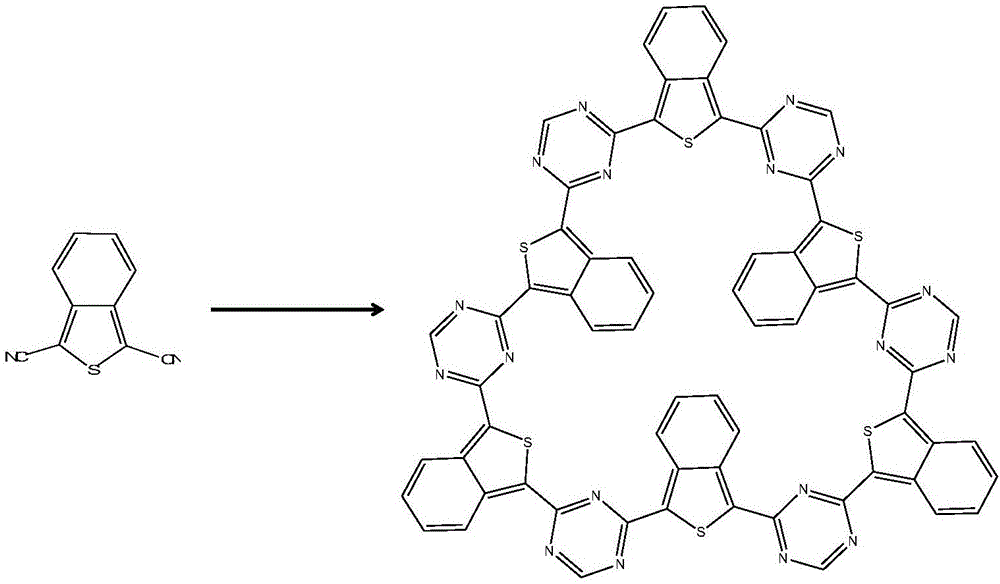
[0033] The framework material used for carbon dioxide adsorption and separation prepared in this example is a covalent triazine framework material based on benzothiophene.
[0034] Its preparation method is as follows: zinc nitrate and 1,3-dicyanobenzothiophene are mixed according to the molar ratio of 1:1, and reacted at a temperature of 200°C for 0.5h to obtain the product.
[0035] It has been determined that the specific surface area of the framework material prepared in this example is 100-1000 m 2 / g, the pore volume is 0.05~0.6cm 3 / g, the microporosity is 0.5-0.9.
Embodiment 2
[0037] The framework material used for carbon dioxide adsorption and separation prepared in this example is a covalent triazine framework material based on benzothiophene.
[0038] Its preparation method is as follows:
[0039] (1) Using the solution mixed with zinc nitrate and zinc sulfate according to the volume ratio of 1:3 as the catalyst, the catalyst of this embodiment and 1,3-dicyanobenzothiophene are mixed according to the molar ratio of 1:1, and the state, heated to 600°C, and reacted for 0.5h to obtain a black solid;
[0040] (2) Grinding the black solid obtained in step (1), and then washing with water, hydrochloric acid solution and methanol solution respectively, to obtain final product.
[0041] It has been determined that the specific surface area of the framework material prepared in this example is 100-1000 m 2 / g, the pore volume is 0.05~0.6cm 3 / g, the microporosity is 0.5-0.9.
Embodiment 3
[0043] The framework material used for carbon dioxide adsorption and separation prepared in this example is a covalent triazine framework material based on benzothiophene.
[0044] Its preparation method is as follows:
[0045] (1) Mix zinc sulfate and 1,3-dicyanobenzothiophene at a molar ratio of 1:3, heat to 400°C under vacuum, and react for 10 hours to obtain a black solid;
[0046] (2) Cool the black solid obtained in step (1) to 40° C., grind it, wash it with water and hydrochloric acid solution three times, wash it with water and methanol solution once, and dry it.
[0047] It has been determined that the specific surface area of the framework material prepared in this example is 100-1000 m 2 / g, the pore volume is 0.05~0.6cm 3 / g, the microporosity is 0.5-0.9.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com