Method for preparing 2,5-dimethylfuran by catalyzing 5-hydroxymethylfurfural
A technology of hydroxymethylfurfural and dimethylfuran, which is applied in the field of organic synthesis, can solve the problems of low selectivity and low activity of non-precious metal catalysts, and achieve high catalytic activity and selectivity, simple production and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
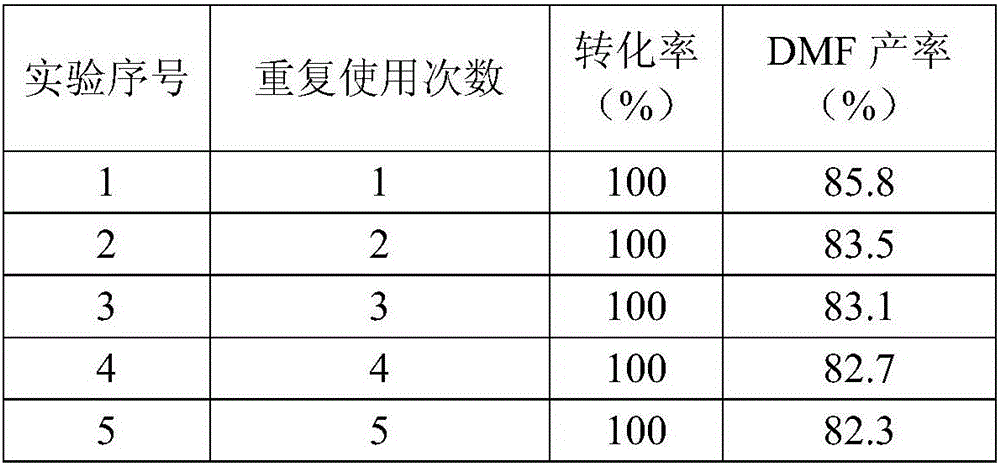
Examples
Embodiment 1
[0039] This embodiment provides a heterogeneous iron-based catalyst, and the raw material components for preparing the heterogeneous iron-based catalyst include ferrous acetate, 1,10-phenanthroline, and activated carbon;
[0040] Wherein, in the above-mentioned heterogeneous iron-based catalyst, the mass percentage content of iron is 3% based on iron element.
[0041] This embodiment also provides a method for preparing the above-mentioned heterogeneous iron-based catalyst, which includes the following steps:
[0042] Mix ferrous acetate and 1,10-phenanthroline with ethanol to dissolve, stir at room temperature for half an hour, then add activated carbon and heat to 60°C for 15 hours, then use a rotary evaporator to spin dry the solvent, and use an oven at 60°C Drying for 8 hours, followed by calcination at 800°C under the protection of an inert gas, to prepare a heterogeneous iron-based catalyst;
[0043] This embodiment also provides a method for preparing 2,5-dimethylfuran...
Embodiment 2
[0047] This embodiment provides a heterogeneous iron-based catalyst, and the raw material components for preparing the heterogeneous iron-based catalyst include hematin and activated carbon;
[0048]Wherein, in the above-mentioned heterogeneous iron-based catalyst, the mass percentage of iron is 3% based on iron element, and the nitrogen-containing compound in this embodiment adopts hematin, which itself contains Fe, so this group of heterogeneous iron is prepared The catalyst does not require the addition of iron compounds.
[0049] This embodiment also provides a method for preparing the above-mentioned heterogeneous iron-based catalyst, which includes the following steps:
[0050] Dissolve hematin with ethanol, stir at room temperature for half an hour, then add activated carbon and heat to 60°C for 15 hours, then use a rotary evaporator to spin dry the solvent, and use an oven to dry at 60°C for 8 hours, then in an inert gas atmosphere Calcining at a temperature of 800°C ...
Embodiment 3
[0055] The preparation of the heterogeneous iron-based catalyst is the same as in Example 1.
[0056] This embodiment also provides a method for preparing 2,5-dimethylfuran from 5-hydroxymethylfurfural catalyzed by the above-mentioned heterogeneous iron-based catalyst, which includes the following steps:
[0057] Step 1, adding 0.5 mmol of 5-hydroxymethylfurfural to 20 mL of n-hexane to prepare a 5-hydroxymethylfurfural solution;
[0058] Step 2, mix the above-mentioned 5-hydroxymethylfurfural solution and 0.1g of the above-mentioned heterogeneous iron-based catalyst in a closed high-pressure reactor, replace the air with hydrogen for 3-4 times, and then fill with hydrogen, and put it under a high pressure of 1MPa The mixture was heated to 240° C. and stirred for 12 hours to carry out hydrodeoxygenation reaction. It was detected by gas chromatography and gas chromatography-mass spectrometry that the conversion rate of HMF was 100%, and the yield of the product DMF was 45.5%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com