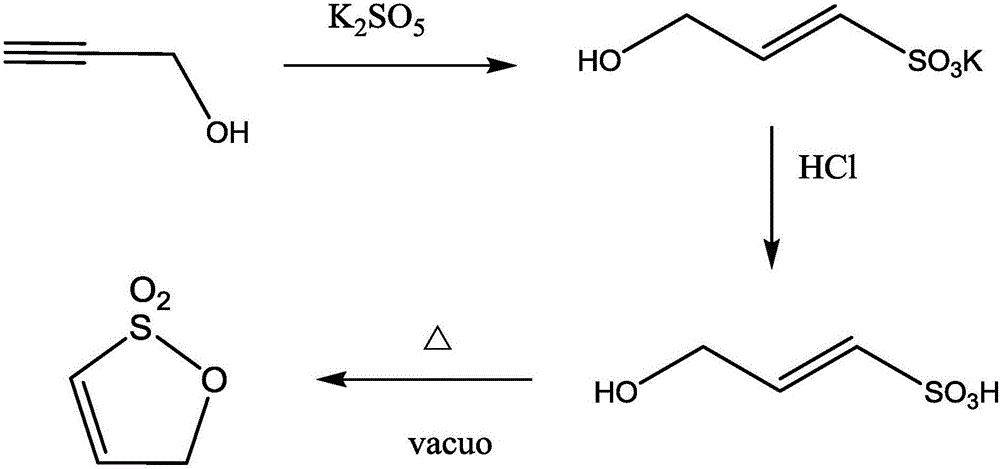
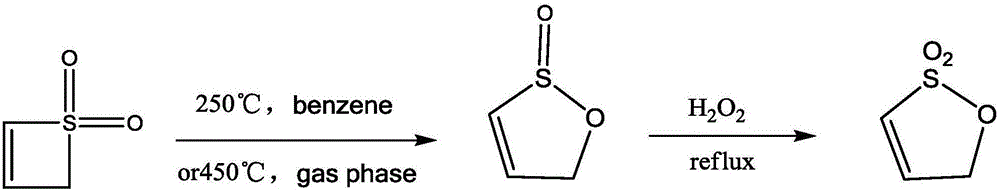
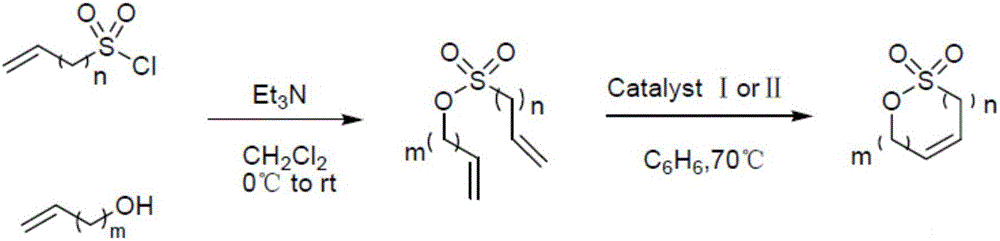Preparation method of allyl-1,3-sultone
A technology of sultone and propane sultone, which is applied in the field of organic compound preparation, can solve the problems of high equipment requirements, difficult industrialization, and more three wastes, and achieve the effect of low equipment requirements, less three wastes, and good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The synthetic route of preparation method of the present invention is as Image 6 Shown: Weigh 10kg of electronic grade 1,3-propane sultone into a jacketed metering tank, heat it at 50°C after dissolving with steam, dissolve 15kg of NBS and 0.05kg of AIBN with 10kg of dichloromethane, and put the microchannel reactor or tubular Preheat the reactor to a certain temperature, pump 1,3-propane sultone at a rate of 2kg / h, pump a mixture of NBS, AIBN, and methylene chloride at a rate of 5kg / h, and simultaneously adjust the outlet back pressure valve to control The reaction pressure was 0.6MPa, the collected reaction solution was filtered to remove succinimide, and the solvent dichloromethane was evaporated under normal pressure to obtain 15kg of 2-bromo-1,3-propane sultone, which was recovered and used mechanically. The rate reached 89.4%.
[0051] Weigh 2kg of 2-bromo-1,3-propane sultone obtained from the above reaction, 0.01kg of polymerization inhibitor, and 30kg of dichl...
Embodiment 2
[0053] Weigh 1.20kg of electronic grade 1,3-propane sultone into a jacketed metering tank, turn on steam to dissolve and keep warm at 50°C, dissolve 30kg of NBS and 1kg of AIBN with 30kg of dichloromethane, preheat the microchannel reactor or tubular reactor Heat to a certain temperature, pump 1,3-propane sultone at a rate of 2kg / h, pump a mixture of NBS, AIBN, and methylene chloride at a rate of 6.1kg / h, and adjust the outlet back pressure valve to control the reaction pressure 1.2MPa, collect the reaction liquid and filter to remove succinimide, evaporate the solvent dichloroethane under normal pressure to get 32kg of 2-bromo-1,3-propane sultone, recover the dichloromethane and apply it mechanically, the bromination reaction yield up to 95%.
[0054] Weigh 2.2kg of 2-bromo-1,3-propane sultone obtained from the above reaction, 0.02kg of polymerization inhibitor, and 30kg of dichloroethane into a 50L enamel reactor with a reflux condenser and heat up to reflux, slowly drop 1k...
Embodiment 3
[0056] Weigh 10kg of electronic grade 1,3-propane sultone into a jacketed metering tank, turn on steam to dissolve and keep warm at 50°C, 15kg of Br 2 , Dissolve 0.05kg BPO with 10kg carbon tetrachloride, preheat the microchannel reactor or tubular reactor to a certain temperature, pump 1,3-propane sultone at a speed of 2kg / h, and pump at a speed of 5kg / h Into Br 2 , BPO, carbon tetrachloride mixture, at the same time adjust the outlet back pressure valve, control the reaction pressure 1.5MPa, collect the reaction liquid and filter to remove succinimide, evaporate the solvent under normal pressure to get 15kg2-bromo-1,3-propane The sultone and solvent recovery are applied mechanically, and the bromination reaction yield reaches 89.4%.
[0057] Weigh 2kg of 2-bromo-1,3-propane sultone obtained from the above reaction, 0.01kg of polymerization inhibitor (BHT), and 30kg of dichloromethane into a 50L enamel reactor with a reflux condenser and heat up to reflux, slowly drop Add 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com