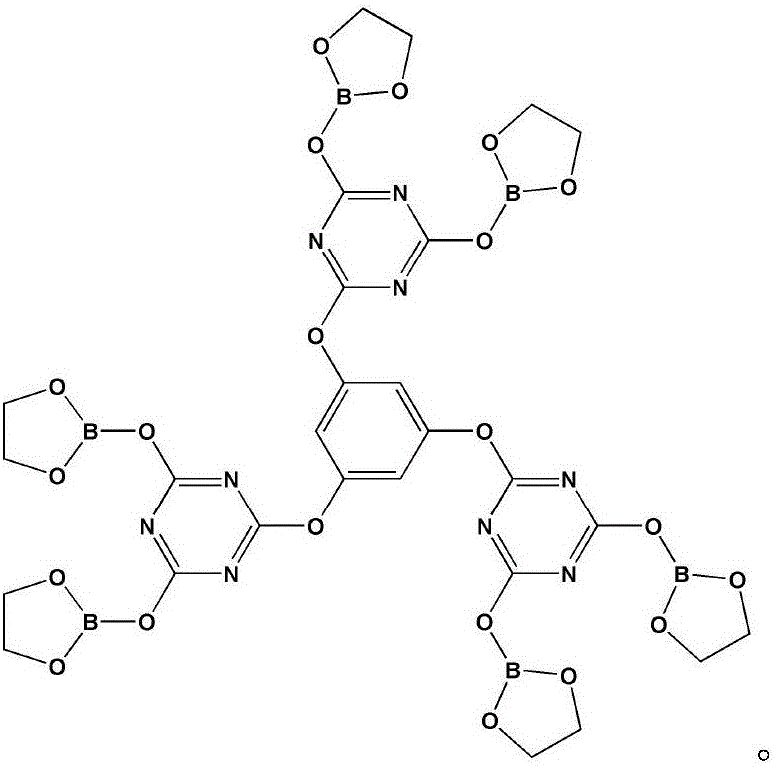
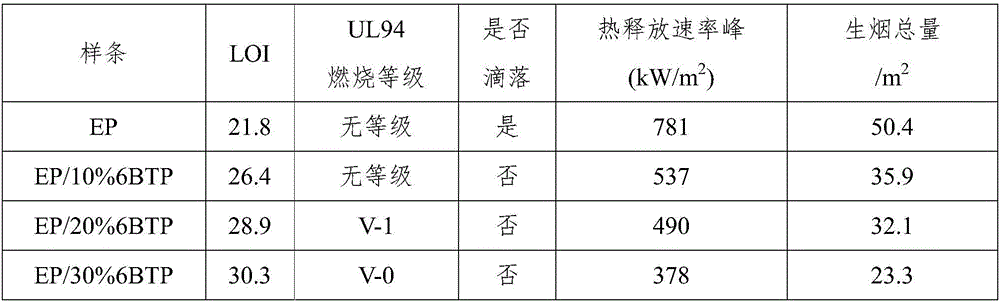
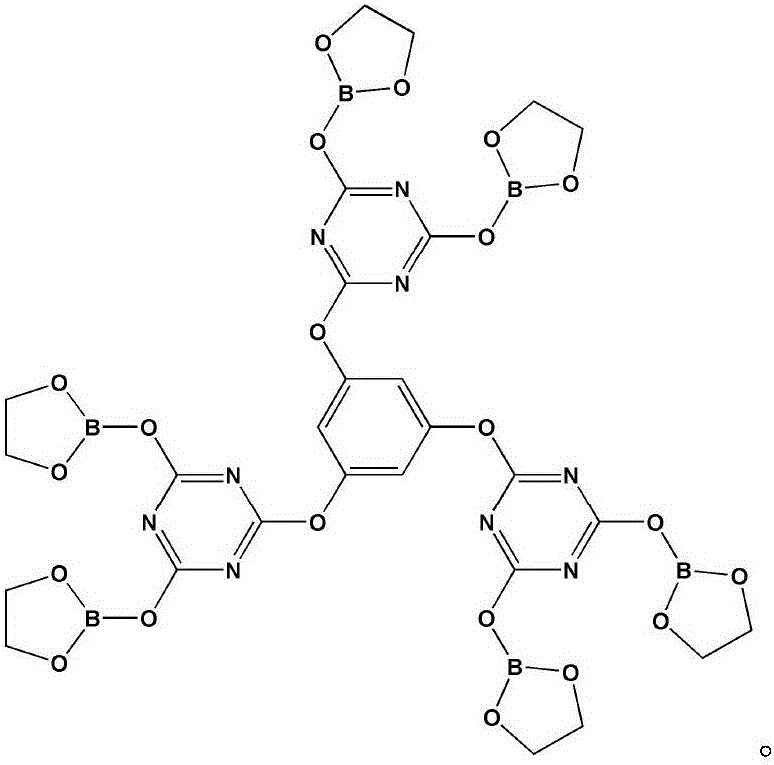
Intumescent flame retardant of star-shaped boric acid ester derivative and preparation method of intumescent flame retardant
A technology of intumescent flame retardant and borate ester, which is applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., can solve the problems of weak flame retardancy and toxicity, and achieve Prevent burning, prevent dripping, and solve the effect of easy dripping
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The first step: 2.58g phloroglucinol and 9.11gN, N-diisopropylethylamine are dissolved in 40ml tetrahydrofuran solution (mixture 1), 16.79g cyanuric chloride is dissolved in 80ml tetrahydrofuran solution, and Fully stir in an ice-water bath for 31 minutes (mixed solution 2), add mixed solution 1 dropwise to mixed solution 2, stir at 5°C for 3 hours, and filter;
[0031] The second step: use a rotary evaporator to remove tetrahydrofuran in the filtrate of the second step to obtain a white solid, use a mixture of petroleum ether and acetone (3:1 by volume) as a flushing solution, and separate with a chromatographic column to obtain an intermediate product 1;
[0032] Step 3: Add 7.5ml of ethylene glycol and 20ml of toluene (as a water-carrying agent) solution into a three-necked flask, stir and gradually heat up to 42°C, add 7.34g of boric acid, continue to heat up to 88°C, and stir for 47 minutes until the boric acid is completely Dissolve; continue to react for 2.5 hou...
Embodiment 2
[0040] The first step: 5.23g phloroglucinol and 18.37gN, N-diisopropylethylamine are dissolved in 80ml tetrahydrofuran solution (mixture 1), 35.31g cyanuric chloride is dissolved in 80ml tetrahydrofuran solution, and Fully stir in an ice-water bath for 31 minutes (mixed solution 2), add mixed solution 1 dropwise to mixed solution 2, stir at 5°C for 3 hours, and filter;
[0041] The second step: use a rotary evaporator to remove tetrahydrofuran in the filtrate of the second step to obtain a white solid, use a mixture of petroleum ether and acetone (3:1 by volume) as a flushing solution, and separate with a chromatographic column to obtain an intermediate product 1;
[0042] Step 3: Add 14ml of ethylene glycol and 40ml of toluene (as a water-carrying agent) solution into the three-necked flask, stir and gradually heat up to 42°C, add 14.89g of boric acid, continue to heat up to 88°C, and stir for 47 minutes until the boric acid is completely dissolved ; When the moisture in the...
Embodiment 3
[0046] The first step: 7.76g phloroglucinol and 27.29gN, N-diisopropylethylamine are dissolved in 120ml tetrahydrofuran solution (mixture 1), 49.63g cyanuric chloride is dissolved in 240ml tetrahydrofuran solution, and Fully stir in an ice-water bath for 37 minutes (mixed solution 2), add mixed solution 1 dropwise to mixed solution 2, stir at 3°C for 5 hours, and filter;
[0047] The second step: use a rotary evaporator to remove tetrahydrofuran in the filtrate of the second step to obtain a white solid, use a mixture of petroleum ether and acetone (3:1 by volume) as a flushing solution, and separate with a chromatographic column to obtain an intermediate product 1;
[0048] Step 3: Add 20.5ml of ethylene glycol and 60ml of toluene (as a water-carrying agent) solution into a three-necked flask, stir and gradually heat up to 47°C, add 22.67g of boric acid, continue to heat up to 70°C, and stir for 55 minutes until the boric acid is completely Dissolve; continue to react for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| heat release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com