Intumescent flame retardant of star borate derivatives and preparation method thereof
A technology of intumescent flame retardants and borate esters, applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., can solve the problems of weak flame retardancy and toxicity, and achieve Effects of preventing burning, preventing dripping, and lowering surface temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
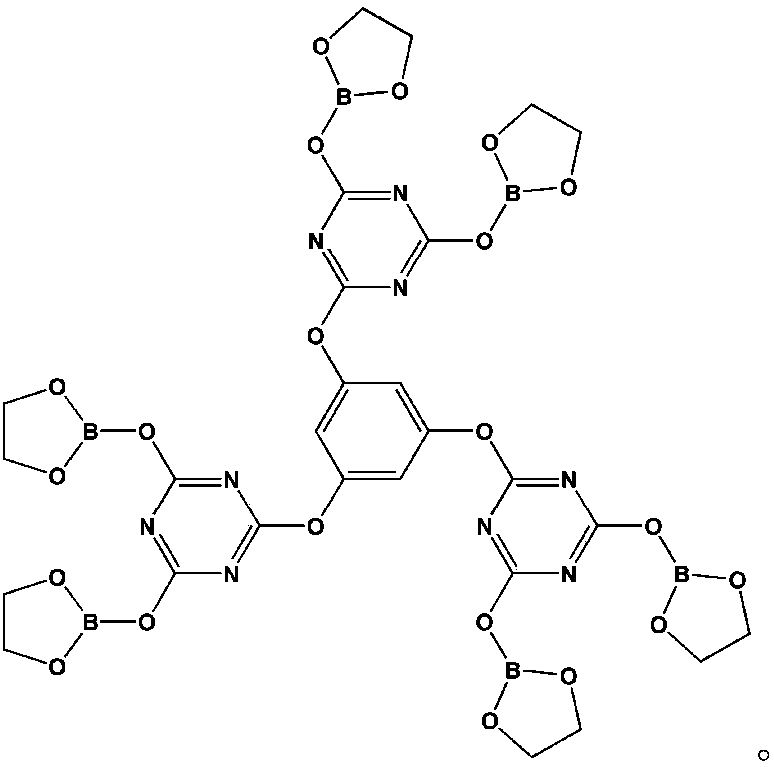
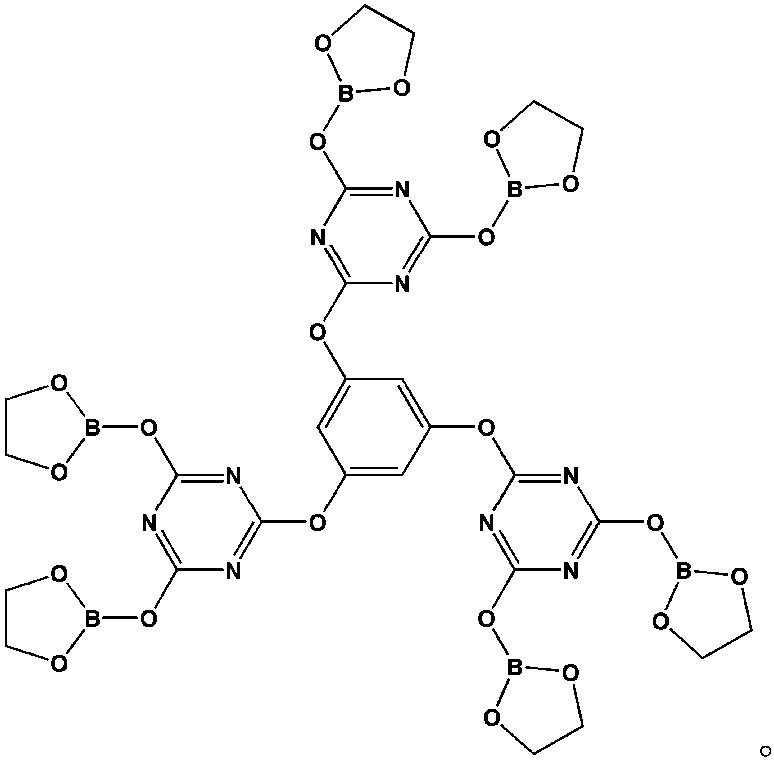
[0030] Step 1: Dissolve 2.58g of phloroglucinol and 9.11g of N,N-diisopropylethylamine in 40ml of tetrahydrofuran solution (mixture 1), dissolve 16.79g of cyanuric chloride in 80ml of tetrahydrofuran solution, and Stir fully in an ice water bath for 31 minutes (mixture 2), add mixture 1 dropwise to mixture 2, stir at 5°C for 3 hours, and filter;
[0031] Step 2: Use a rotary evaporator to remove the tetrahydrofuran in the filtrate of the second step to obtain a white solid. Use a mixture of petroleum ether and acetone (volume ratio of 3:1) as the washing liquid and separate with a chromatographic column to obtain the intermediate product 1;
[0032] The third step: Add 7.5ml ethylene glycol and 20ml toluene (as a water-carrying agent) solution in a three-necked flask, stir and gradually raise the temperature to 42℃, add 7.34g boric acid, continue to raise the temperature to 88℃, and stir for 47 minutes until the boric acid is complete Dissolve; wait for the moisture in the water s...
Embodiment 2
[0040] Step 1: Dissolve 5.23g phloroglucinol and 18.37g N,N-diisopropylethylamine in 80ml tetrahydrofuran solution (mixture 1), dissolve 35.31g cyanuric chloride in 80ml tetrahydrofuran solution, and Stir fully in an ice water bath for 31 minutes (mixture 2), add mixture 1 dropwise to mixture 2, stir at 5°C for 3 hours, and filter;
[0041] Step 2: Use a rotary evaporator to remove the tetrahydrofuran in the filtrate of the second step to obtain a white solid. Use a mixture of petroleum ether and acetone (volume ratio of 3:1) as the washing liquid and separate with a chromatographic column to obtain the intermediate product 1;
[0042] The third step: Add 14ml ethylene glycol and 40ml toluene (as a water-carrying agent) solution in a three-necked flask, stir and gradually raise the temperature to 42℃, add 14.89g boric acid, continue to raise the temperature to 88℃, stir for 47 minutes until the boric acid is completely dissolved ; When the moisture in the water separator no longer...
Embodiment 3
[0046] The first step: Dissolve 7.76g phloroglucinol and 27.29g N,N-diisopropylethylamine in 120ml tetrahydrofuran solution (mixture 1), dissolve 49.63g cyanuric chloride in 240ml tetrahydrofuran solution, and Stir fully in an ice water bath for 37 minutes (mixture 2), add mixture 1 dropwise to mixture 2, stir at 3°C for 5 hours, and filter;
[0047] Step 2: Use a rotary evaporator to remove the tetrahydrofuran in the filtrate of the second step to obtain a white solid. Use a mixture of petroleum ether and acetone (volume ratio of 3:1) as the washing liquid and separate with a chromatographic column to obtain the intermediate product 1;
[0048] The third step: Add 20.5ml ethylene glycol and 60ml toluene (as a water-carrying agent) solution into a three-necked flask, stir and gradually raise the temperature to 47℃, add 22.67g boric acid, continue to raise the temperature to 70℃, and stir for 55 minutes until the boric acid is complete Dissolve; wait for the moisture in the water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| heat release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com