Preparation method of lithium ion conductor coated spinel lithium manganate positive electrode material
A technology of spinel lithium manganese oxide and positive electrode materials, which can be used in battery electrodes, electrical components, circuits, etc., and can solve the problems of poor high-rate performance and poor conductivity of materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
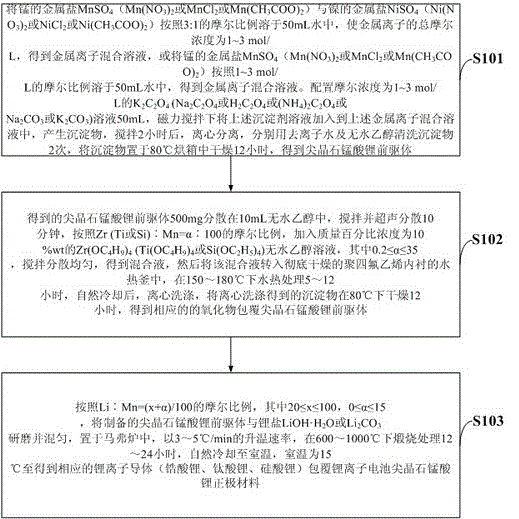
[0043] (1) Manganese metal salt MnSO 4 Metal salt NiSO with nickel 4 Dissolve in 50mL water according to the molar ratio of 3:1, so that the total molar concentration of metal ions is 1 mol / L, to obtain a mixed solution of metal ions. Configure K with a molar concentration of 1 mol / L 2 C 2 o 4 Solution 50mL, under magnetic stirring, add the above precipitant solution into the above metal ion mixed solution to produce a precipitate, stir for 2 hours, centrifuge, wash the precipitate twice with deionized water and absolute ethanol, and place the precipitate Dry in an oven at 80°C for 12 hours to obtain a spinel lithium manganate precursor;
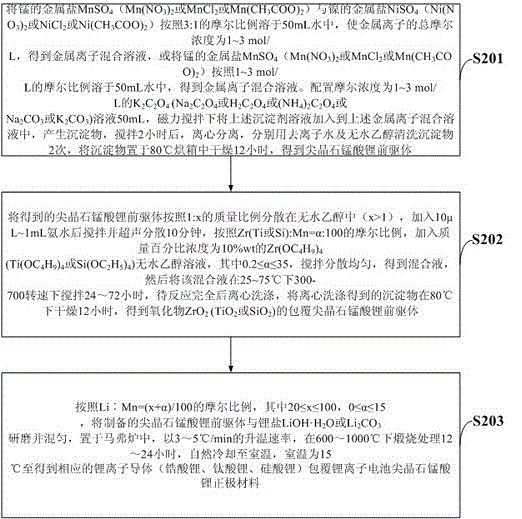
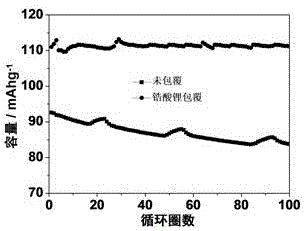
[0044] (2) Disperse the spinel lithium manganate precursor obtained in the above step (1) in absolute ethanol at a mass ratio of 1:10, add 1mL of ammonia water, stir and ultrasonically disperse for 10 minutes, according to Zr:Mn=0.5 : The molar ratio of 100, add the Zr(OC that mass percent concentration is 10%wt 4 h 9 ) 4 Anhydrous e...
Embodiment 2
[0047] (1) Manganese metal salt MnSO 4 Metal salt NiSO with nickel 4 Dissolve in 50mL water according to the molar ratio of 3:1, so that the total molar concentration of metal ions is 2 mol / L, to obtain a mixed solution of metal ions. Configure K with a molar concentration of 2 mol / L 2 C 2 o 4 Solution 50mL, under magnetic stirring, add the above precipitant solution into the above metal ion mixed solution to produce a precipitate, stir for 2 hours, centrifuge, wash the precipitate twice with deionized water and absolute ethanol, and place the precipitate Dry in an oven at 80°C for 12 hours to obtain a spinel lithium manganate precursor;
[0048] (2) Disperse the spinel lithium manganate precursor obtained in the above step (1) in absolute ethanol at a mass ratio of 1:20, add 1mL of ammonia water, stir and ultrasonically disperse for 10 minutes, according to Ti:Mn=0.6 : The molar ratio of 100, add the Ti(OC that mass percent concentration is 10%wt 4 h 9 ) 4 Anhydrous e...
Embodiment 3
[0051] (1) Manganese metal salt Mn(NO 3 ) 2 and nickel metal salt Ni(NO 3 ) 2 Dissolve in 50mL water according to the molar ratio of 3:1, so that the total molar concentration of metal ions is 1.5 mol / L, to obtain a mixed solution of metal ions. Configure Na with a molar concentration of 1.5 mol / L 2 C 2 o 4 Solution 50mL, under magnetic stirring, add the above precipitant solution into the above metal ion mixed solution to produce a precipitate, stir for 2 hours, centrifuge, wash the precipitate twice with deionized water and absolute ethanol, and place the precipitate Dry in an oven at 80°C for 12 hours to obtain a spinel lithium manganate precursor;
[0052] (2) Disperse the spinel lithium manganate precursor obtained in the above step (1) in absolute ethanol at a mass ratio of 1:15, add 1mL of ammonia water, stir and ultrasonically disperse for 10 minutes, according to Si:Mn=0.2 : The molar ratio of 100, add the Si(OC that mass percent concentration is 10%wt 2 h 5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com