Catalyst for carbon dioxide hydrogenation synthesis of low-carbon alcohols, preparation method and application thereof
A carbon dioxide and catalyst technology, applied in the field of catalysts, can solve problems such as low catalyst activity, achieve the effects of high activity, avoid raw material loss, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
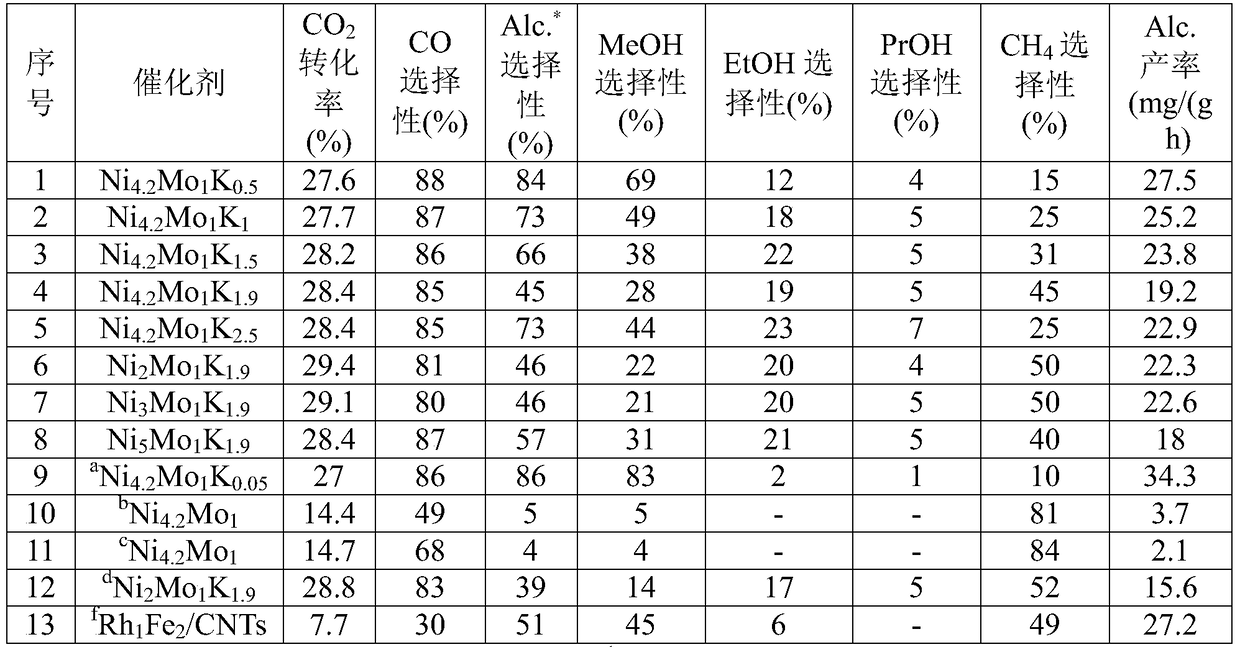
[0024] Ni 4.2 Mo 1 K 0.5 Catalyst preparation and its catalytic performance for carbon dioxide hydrogenation to low-carbon alcohols:
[0025] Take by weighing 12.21g nickel nitrate (Ni(NO 3 )·6H 2 O) was dissolved in 200mL deionized water, under stirring, the rotation speed was 500r / min, heated to 80°C, and 7.25g of anhydrous potassium carbonate (K 2 CO 3 ) was dissolved in 250mL deionized water, and slowly added dropwise to the above-mentioned nickel nitrate solution, and the dripping was completed in about 2 hours. After the obtained precipitate was aged for 24 hours, the supernatant was poured off to obtain a slurry-like precipitate. Heat this slurry precipitate to 80 ℃ under stirring, weigh 1.8g ammonium molybdate ((NH 4 ) 6 Mo 7 o 24 4H 2 O) dissolved in 200mL of deionized water, slowly added dropwise, and continued to stir at 80°C for 5h to allow the two to fully complete the ion exchange. Suction filtration, the obtained solid was dried at 60°C for 12h, and ...
Embodiment 2
[0028] Ni 4.2 Mo 1 K 1 Catalyst preparation and its catalytic performance for carbon dioxide hydrogenation to low-carbon alcohols:
[0029] The catalyst preparation process and activity evaluation were the same as in Example 1, except that the amount of potassium carbonate was changed to 0.44g during impregnation. At 5.0MPa, 320°C, V(H 2 ) / V(CO 2 ) / V(Ar)=72 / 24 / 4, under the reaction conditions of GHSV=3000mL / (h g), Ni 4.2 Mo 1 K 1 CO on catalyst 2 The conversion rate was 27.7%, the selectivity of total alcohol was 73% (excluding CO), wherein the selectivity of ethanol was 18%, and the space-time yield of total alcohol was 25.2 mg / (h g). The detailed results are shown in Table 1, Sequence 2.
Embodiment 3
[0031] Ni 4.2 Mo 1 K 1.5 Catalyst preparation and its catalytic performance for carbon dioxide hydrogenation to low-carbon alcohols:
[0032] The catalyst preparation process and activity evaluation were the same as in Example 1, except that the amount of potassium carbonate was changed to 0.67g during impregnation. At 5.0MPa, 320°C, V(H 2 ) / V(CO 2 ) / V(Ar)=72 / 24 / 4, under the reaction conditions of GHSV=3000mL / (h g), Ni 4.2 Mo 1 K 1.5 CO on catalyst 2 The conversion rate reached 28.2%, the selectivity of total alcohol was 66% (excluding CO), wherein the selectivity of ethanol was 22%, and the space-time yield of total alcohol was 23.8 mg / (h g). The detailed results are shown in Table 1, Sequence 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com