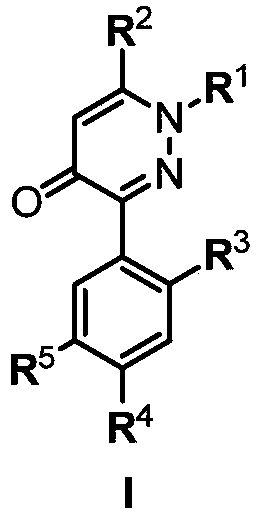
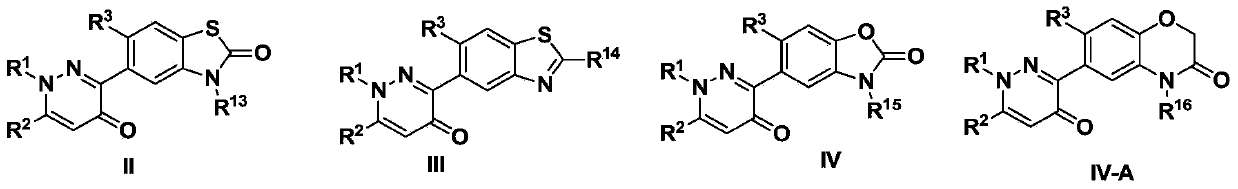
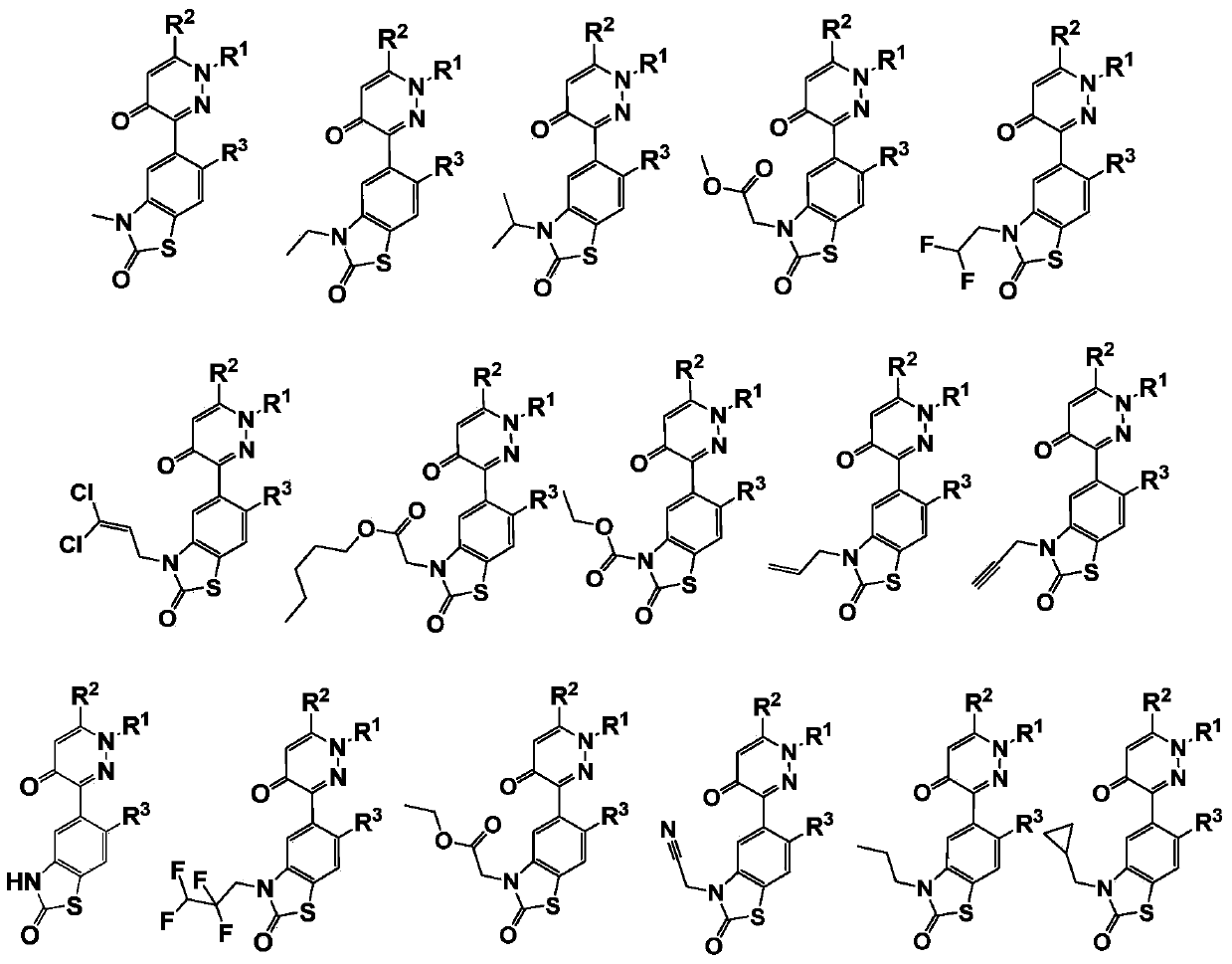
3-arylpyridazinone compound, preparation method, pesticide composition and application
A compound and technology of pyridazinone, applied in the field of 3-aryl pyridazinone compounds, can solve the problems of poor ecotoxicity and environmental compatibility, low herbicidal activity of herbicides, narrow herbicidal spectrum and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] intermediate synthesis
[0151] Synthesis of 3-chloro-1-methyl-6-trifluoromethylpyridazin-4(1H)-one
[0152]
[0153] 300g (3.04mol, 1eq.) of maleic anhydride was suspended in 900mL of carbon tetrachloride, and then 513g (3.2mol, 1.05eq.) of liquid bromine was slowly added dropwise under light, and after the addition of liquid bromine was completed, the reaction was 32 -40h, proton nuclear magnetic spectrum tracking reaction process. After the reaction was completed, the solvent carbon tetrachloride and excess liquid bromine were distilled off under reduced pressure to obtain 750 g of light yellow solid 2,3-dibromosuccinic anhydride 2 with a yield of 95%.
[0154] 1 H-NMR (300MHz, CDCl 3 ):δ4.88(s,2H)
[0155] Weigh 300g (1.16mol, 1eq.) of compound 2 in a 2L three-neck flask, add 500mL of glacial acetic acid at room temperature to dissolve 2, slowly add 105g (1.28mol, 1.1eq.) of anhydrous sodium acetate, and reflux for 5h. After the reaction was completed, the r...
Embodiment 2
[0170] FQ-71
[0171]
[0172] 2.9g (73.2mmol, 1.2eq.) of sodium hydroxide was dissolved in 200ml of water, and 10.0g (61mmol, 1.0eq.) of compound 1-2 was added in batches to aqueous sodium hydroxide solution. 6.9g (73.2mmol, 1.2eq.) of methyl chloroformate, after dropping, warmed up to room temperature and reacted for 3 hours. After the reaction was complete, it was suction filtered, and the filter cake was dried to obtain 12.7 g of white solid 2-2, with a yield of 93.7%.
[0173] 10.0g (45.2mmol, 1.0eq.) of compound 2-2 was dissolved in 40ml of 98% concentrated sulfuric acid, cooled to 0°C in an ice-water bath, and 3.0g (47.4mmol, 1.05eq.) of 65% concentrated nitric acid was added dropwise. React for 5 hours. After the reaction was complete, the reaction solution was slowly poured into ice water, a yellow solid precipitated out, filtered with suction, and the filter cake was dried to obtain 9.2 g of yellow solid 3-2 with a yield of 76.4%.
[0174] 10.0g (37.6mmol, 1.0e...
Embodiment 3
[0181] FQ-67
[0182]
[0183] 339mg (1.0mmol, 1.0eq.) Compound FQ-71 was dissolved in 40ml of acetonitrile, 207mg (1.5mmol, 1.5eq.) of potassium carbonate was added, 255mg (1.5mmol, 1.5eq.) of iodoisopropane was added, and the reaction was carried out at 50°C 4 hours. After the reaction was complete, cool, filter with suction, and spin dry the filtrate to obtain a crude product, which was purified by column chromatography to obtain 320 mg of FQ-67 with a yield of 83.9%.
[0184] Solid, m.p.: 99-100°C; HPLC: 99.4%; 1 H-NMR (300MHz, CDCl 3 ):δ7.49(s,1H),6.99(d,2H),4.43-4.60(m,1H),4.08(s,3H),1.38(d,6H); 19 F-NMR (282MHz, CDCl 3 ):δ-70.64(s,3F); MS(ESI):m / z 380(M + ),403(M+Na) +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com