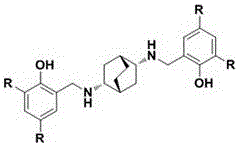
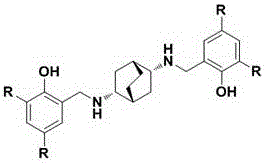
Novel synthesis method of florfenicol
A technology of florfenicol and synthetic method, which is applied in the field of florfenicol synthesis, can solve the problems of low total yield, long route, high waste water treatment cost, etc., and achieve the goal of saving treatment cost, simple reaction steps, and reduced cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
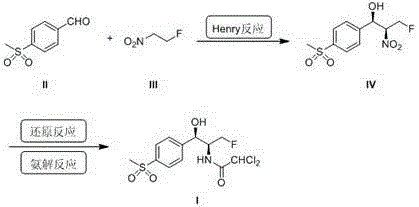
[0030] Example 1 Preparation of (1R, 2S)-3-fluoro-1-(4-(methylsulfonyl)benzene)-2-nitropropan-1-ol (IV)
[0031] Step 1 Preparation of catalyst solution: 3Å molecular sieve, tetrahydro-salen ligand (Ia) (82 mg, 0.2 mmol), (CuOTf) 2 .C 6 H 5 CH 3 (10 mg, 0.02 mmol) was added to dichloromethane (8 mL) to prepare a catalytic solution; the added amount of the tetrahydro-salen ligand (Ia) was 10 mol of the added amount of methylsulfonyl benzaldehyde (II) %; the CuOTf) 2 .C 6 H 5 CH 3 The added amount accounts for 1 mol% of the added amount of methylsulfonyl benzaldehyde (II).
[0032] Step 2 Asymmetric Henry reaction: Add fluoronitroethane (III) (2.1 g, 0.022 mol) to the prepared catalyst solution, stir at room temperature for 10 minutes, add p-methylsulfonyl benzaldehyde (II) (3.7 g, 0.02 mol), heated to 30°C and stirred for 24 hours to perform catalytic reaction.
[0033] Step 3 Collection: Distill the materials after the catalytic reaction under reduced pressure and column chromatogr...
Embodiment 2
[0035] Example 2 Preparation of (1R,2S)-3-fluoro-1-(4-(methylsulfonyl)benzene)-2-nitropropane-1-ol (IV)
[0036] Step 1 Preparation of catalyst solution: 3Å molecular sieve, tetrahydro-salen ligand (Ib) (93 mg, 0.2 mmol), (CuOTf) 2 .C 6 H 5 CH 3 (10 mg, 0.02 mmol) was added to tetrahydrofuran (8 mL) in sequence to prepare a catalyst solution; the added amount of tetrahydro-salen ligand (Ia) was 10 mol% of the added amount of methylsulfonyl benzaldehyde (II) ; The CuOTf) 2 .C 6 H 5 CH 3 The added amount accounts for 1 mol% of the added amount of methylsulfonyl benzaldehyde (II)
[0037] Step 2 Asymmetric Henry reaction: Add fluoronitroethane III (2.1 g, 0.022 mol) to the catalyst solution, stir at room temperature for 10 minutes, add p-methylsulfonyl benzaldehyde II (3.7 g, 0.02 mol), and heat The reaction was stirred at 25°C for 24 hours, and the reaction was complete.
[0038] Step 3 Collection: The mixture after the catalytic reaction is subjected to vacuum distillation, and colu...
Embodiment 3
[0040] Example 3 Preparation of (1R, 2S)-3-fluoro-1-(4-(methylsulfonyl)benzene)-2-nitropropan-1-ol (IV)
[0041] Step 1 Preparation of catalyst solution: 3Å molecular sieve, tetrahydro-salen ligand (Ic) (104 mg, 0.2 mmol), (CuOTf) 2 .C 6 H 5 CH 3 (10 mg, 0.02 mmol) was added to toluene (8 mL) in sequence to prepare a catalytic solution; the added amount of tetrahydro-salen ligand (Ia) was 10 mol% of the added amount of methylsulfonyl benzaldehyde (II) ; The CuOTf) 2 .C 6 H 5 CH 3 The added amount accounts for 1 mol% of the added amount of methylsulfonyl benzaldehyde (II).
[0042] Step 2 Asymmetric Henry reaction: add fluoronitroethane III (2.1 g, 0.022 mol) to the catalyst solution, stir at room temperature for 10 minutes, add p-methylsulfonyl benzaldehyde II (3.7 g, 0.02 mol), and heat Stir and react at 35°C for 24 hours, and the reaction is complete.
[0043] Step 3 Collection: The materials after the catalytic reaction are subjected to vacuum distillation, and column chromatogr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com