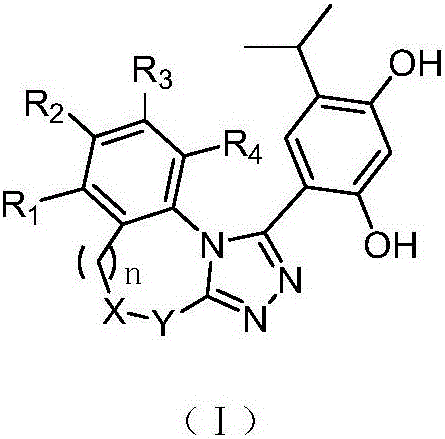
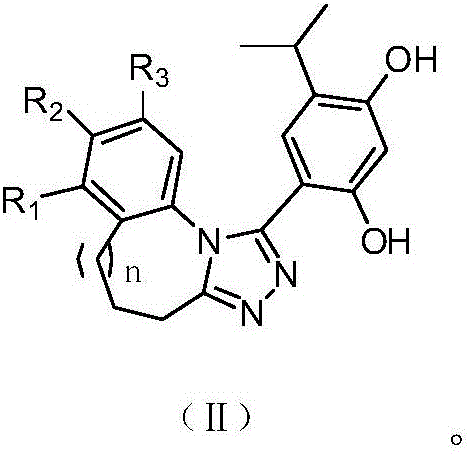
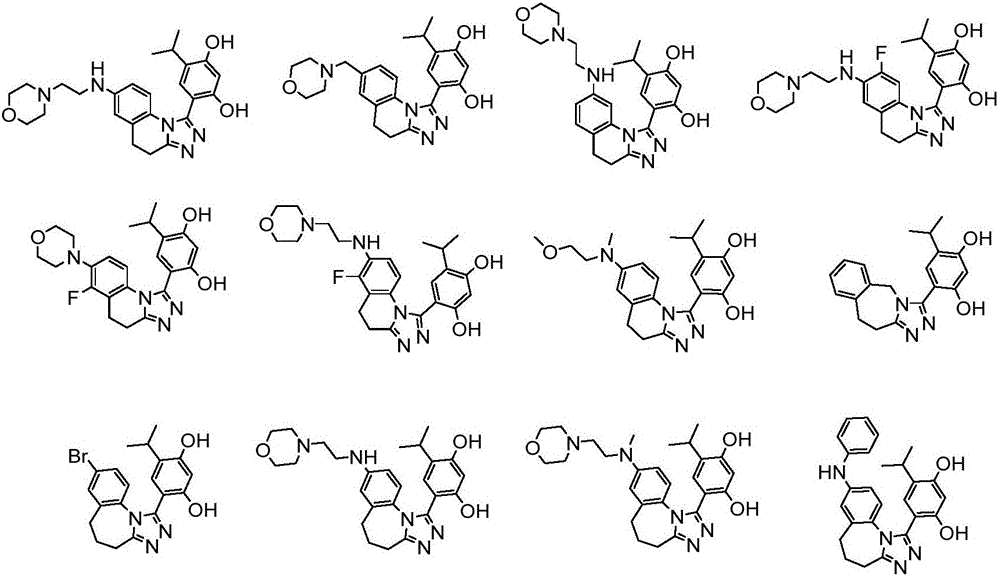
Triazole derivative having HSP90 (Heat Shock Protein) inhibiting activity, as well as preparation method and application of triazole derivative
A technology of triazole derivatives and stereoisomers, applied in the field of triazole derivatives and their preparation, can solve the problems of preventing clinical development and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] 4-(7-Bromo-4,5-dihydro-[1,2,4]triazol[4,3-a]quinolin-1-yl)-6-isopropylbenzene-1,3-di phenol
[0125]
[0126] Step 1: 6-bromo-3,4-dihydroquinolin-2(1H)-one
[0127] Compound 3,4-dihydro-2(1H)-quinolinone (10.0 g, 67.95 mmol) was dissolved in N,N-dimethylformamide (100 mL). After cooling to 0°C, N-bromosuccinimide (12.7 g, 71.34 mmol) was added in batches, and the reaction was stirred for 6 hours while slowly raising the temperature. The reaction solution was concentrated to dryness, added with ethyl acetate, washed with sodium bicarbonate solution and brine successively. The organic phase was dried, filtered and concentrated to dryness to obtain 6-bromo-3,4-dihydroquinolin-2(1H)-one (14g) with a yield of 90%.
[0128] Step 2: 6-bromo-3,4-dihydroquinoline-2(1H)-thione
[0129] Compound 6-bromo-3,4-dihydroquinolin-2(1H)-one (10.0g, 44.23mmol) was dissolved in toluene (100mL), Lawson's reagent (8.95g, 22.12mmol) was added, and the suspension The solution was heated...
Embodiment 2
[0162] 4-isopropyl-6-(7-((2-morpholinoethyl)amine)-4,5-dihydro-[1,2,4]triazol[4,3-a]quinoline- 1-yl)benzene-1,3-diol
[0163]
[0164] Step 1: 1-(5-isopropyl-2,4-dimethoxybenzene)-N-(2-morpholinoethyl)-4,5-dihydro-[1,2,4]triazole [4,3-a]quinolin-7-amine
[0165] The compound 7-bromo-1-(5-isopropyl-2,4-dimethoxyphenyl)-4,5-dihydro-[1,2,4]triazol[4,3-a] Quinoline (70 mg, 0.16 mmol), 2-morpholinoethyl-1-amine (43 mg, 0.33 mmol), tris(dibenzylideneacetone)dipalladium (15 mg, 0.013 mmol), 4,5-diphenylene Phosphine-9,9-dimethylxanthene (15 mg, 0.013 mmol) and sodium tert-butoxide (35 mg, 0.36 mmol) were dissolved in anhydrous toluene (2 mL). Under the protection of nitrogen, microwave heating to 100° C. was performed for 2 hours. Concentrated to dryness under reduced pressure, the crude product was separated by column chromatography (dichloromethane / methanol=100:0~90:10, v / v) to obtain 1-(5-isopropyl-2,4-dimethoxybenzene) -N-(2-morpholinoethyl)-4,5-dihydro-[1,2,4]triazol[4,3...
Embodiment 3
[0172] 4-isopropyl-6-(7-(morpholinylmethyl)-4,5-dihydro-[1,2,4]triazol[4,3-a]quinolin-1-yl)benzene- 1,3-diphenol
[0173]
[0174] Step 1: 2-oxo-1,2,3,4-tetrahydroquinoline-6-carbaldehyde
[0175] Under nitrogen protection, in a 100mL three-necked flask, the compound 6-bromo-3,4-dihydroquinolin-2(1H)-one (2.5g, 11.06mmol) and N,N-dimethylformamide (2.5g , 11.06 mmol) was dissolved in dry tetrahydrofuran (50 mL) and cooled to -78 °C. Slowly add tert-butyllithium solution (24.1mL, 38.7mmol, 1.6M in pentane) dropwise, continue to react at -78°C for 4 hours after the dropwise addition, add acetic acid (2.5mL, slowly warm up to room temperature. Diluted with ethyl ester (100mL), washed successively with saturated sodium bicarbonate solution and brine. The organic phase was dried, filtered and concentrated to dryness to obtain 2-oxo-1,2,3,4-tetrahydroquinoline-6-carbaldehyde (0.6 g), yield 30%.
[0176] ESI-MS m / z: 176.2.
[0177] Step 2: 6-(morpholinylmethyl)-3,4-dihydroqui...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com