Method for selectively precipitating and separating cobalt in potential-control manner
A technology of precipitation separation and selectivity, applied in the field of hydrometallurgical process, can solve the problems of long process flow, difficult separation and extraction, difficult to use, etc., and achieve the effect of simple process, stable technical indicators and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
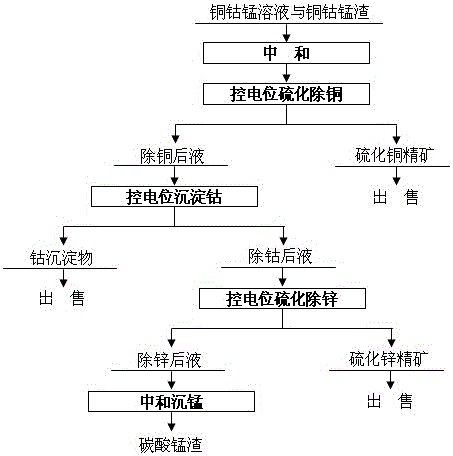
[0027] The copper-cobalt-manganese solution and copper-cobalt-manganese slag in the extraction and removal process of the cobalt smelting system, the main composition range of the copper-cobalt-manganese solution is (g / L): Cu15.4, Co2.2, Zn9.8, Ca6.24, Mn105. 2. Cl180 and H4.5, the main composition range of copper, cobalt and manganese slag is (%): Cu17.5, Zn4.3, Co0.8 and Mn22.8. Sodium sulfide, hydrochloric acid and ethyl sodium xanthate are all industrial grade reagents, the mass percentage of sodium sulfide is not less than 60.0%, the mass percentage of hydrochloric acid is not less than 31.0% and the mass percentage of ethyl sodium xanthate is not less than 82.0%.
[0028] First neutralize the copper-cobalt-manganese solution with copper-cobalt-manganese slag and control the final pH of the mixed solution to 0.4, then keep the solution temperature at 45°C and the stirring speed at 120r / min, then add sodium sulfide solution with a concentration of 234g / L, and add industrial...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com