Troponin I monoclonal antibody magnetic particles and preparation method thereof, and detection kit
A monoclonal antibody and detection kit technology, applied in the field of in vitro diagnostic medical testing, can solve the problems of unfavorable promotion of troponin I detection, high cost, poor repeatability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
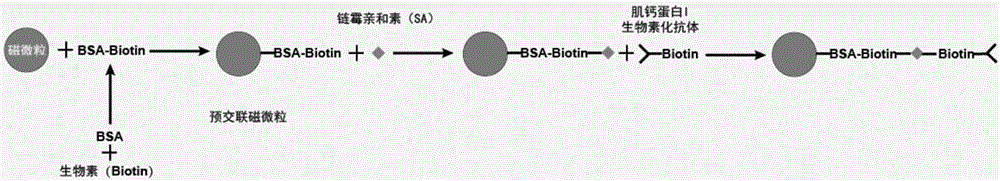
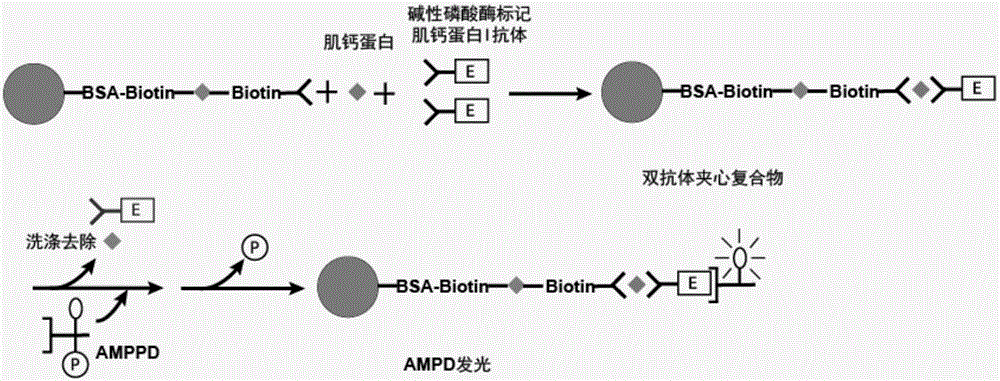
[0063] Correspondingly, the present invention also provides a method for preparing the troponin I monoclonal antibody magnetic particles described above, the method comprising the following steps:
[0064] 1) Using biotin derivatives to pre-crosslink magnetic particles;
[0065] 2) coating the biotin derivative pre-crosslinked magnetic particles obtained in step 1) with streptavidin; and
[0066] 3) Blocking the magnetic particles obtained in step 2), and then reacting with troponin I monoclonal antibody-biotin to prepare troponin I monoclonal antibody magnetic particles.
[0067] In specific embodiments, the method may include:
[0068] a) using biotin derivatives to pre-crosslink the magnetic particles, and then coating them with streptavidin;
[0069] b) reacting the magnetic particles treated in step a) with troponin I monoclonal antibody-biotin after being blocked;
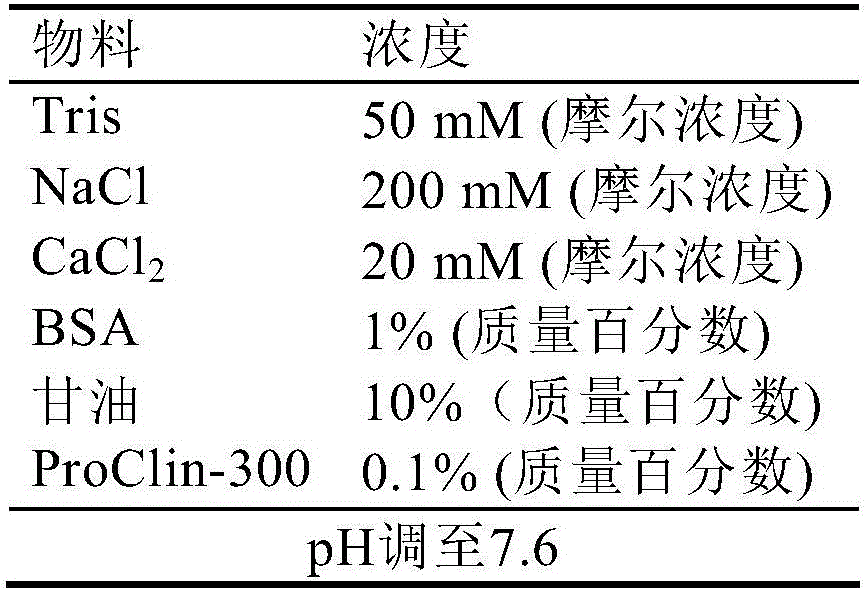
[0070] c) Preserving the magnetic particles treated in step b) in a magnetic particle preservation solu...
Embodiment 1
[0142] Example 1. Preparation of Troponin I Monoclonal Antibody Magnetic Particles
[0143] 1) Dissolve and dilute BSA with 10mM PBS to 2mg / mL, add 2mg / mL Biotin aqueous solution to the 2mg / mL BSA solution according to the molar concentration ratio of 1:20, and mix well;
[0144] 2) React the mixture at room temperature for 30 minutes, place it at 2-8°C, and dialyze with 10mM PBS for 16-24 hours;
[0145] 3) Take out the dialysate and dilute BSA-Biotin to 1mg / mL with 10mM PBS;
[0146] 4) Take 10 mg of 1.0 μm magnetic particles whose active functional groups are carboxyl groups, and wash them twice with 50 mM MES buffer at pH 6.0;
[0147] 5) Remove the supernatant after magnetic suction, add 0.5mL of 50mM MES buffer solution with pH 6.0 and mix well, then add 0.5mL of 25mg / mL carbodiimide (EDC) solution, mix well, and react at room temperature for 30 minutes;
[0148] 6) Wash twice with a solution containing 50 mM pH 7.8 Tris-HCl, 150 mM NaCl, and 1% BSA;
[0149] 7) Add 4...
Embodiment 2
[0161] Example 2. Sensitivity Detection of Troponin I Monoclonal Antibody Magnetic Particles
[0162] Using the troponin I monoclonal antibody magnetic particles obtained in Example 1, the sensitivity detection was carried out as described in the materials and methods, and the results are shown in the following table:
[0163]
[0164]
[0165] Note:
[0166] (1) Magnetic particles obtained by directly cross-linking troponin I monoclonal antibody;
[0167] (2) Traditional streptavidin-biotin troponin I monoclonal antibody magnetic particles;
[0168] (3) The multistage amplified streptavidin-biotin troponin I monoclonal antibody magnetic particle of the present invention.
[0169] As can be seen from the above table, using the multistage amplified streptavidin-biotin troponin I monoclonal antibody magnetic particles of the present invention, its sensitivity (S / N value) is higher than that of direct cross-linking and traditional streptavidin - The biotin method improve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com