A quantitative detection method for the titer of a swine fever neutralizing antibody in swine serum and a detection kit
A quantitative detection method and antibody titer technology, applied in measuring devices, instruments, scientific instruments, etc., can solve the problems of high professional skill requirements for operators, high cost of manpower and material resources, and restrictions on popularization and application, and achieve wide application value, The effect of low cost and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
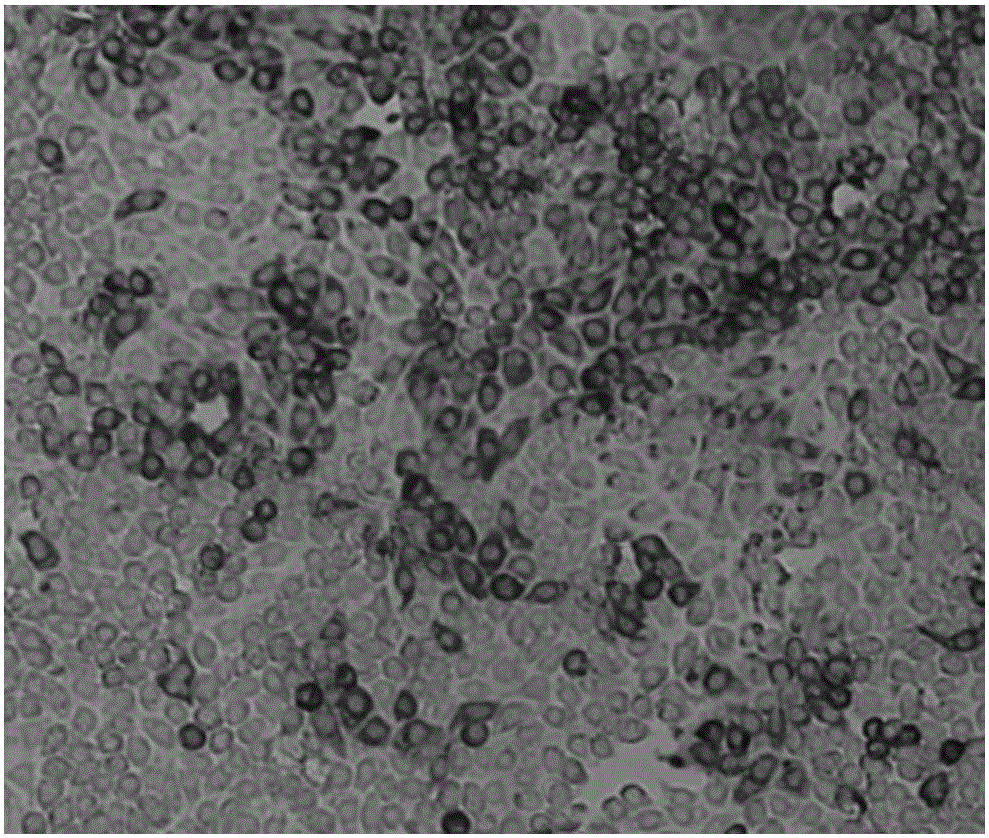
[0052] Embodiment 1, preparation swine fever rabbitization attenuated cytotoxicity (neutralization poison) and TCID thereof 50 determination
[0053] 1. Virus cultivation and harvesting
[0054] Inoculate the monolayer of swine testis (Swine Testis, ST) cells (derived from ATCC) with 0.3% (mass volume ratio, W / V) of classical swine fever rabbit attenuated spleen gonorrhea (purchased from the Central Prison Institute), and use the maintenance solution MEM culture medium (purchased from Yixing Saier Biotechnology Co., Ltd.), newborn bovine serum (purchased from Inner Mongolia Jinyuankang Co., Ltd.) with a content of 1%-3% (V / V), harvested the cell culture after culturing at 37°C for 4 days, and recorded For 1 harvest, the 1 harvest culture was frozen and thawed three times and then inoculated on ST monolayer cells at 10% (V / V), cultured at 37°C for 4 days, and the cell culture was harvested, which was recorded as 2 harvests, and the 2 harvest cultures were After freezing and t...
Embodiment 2
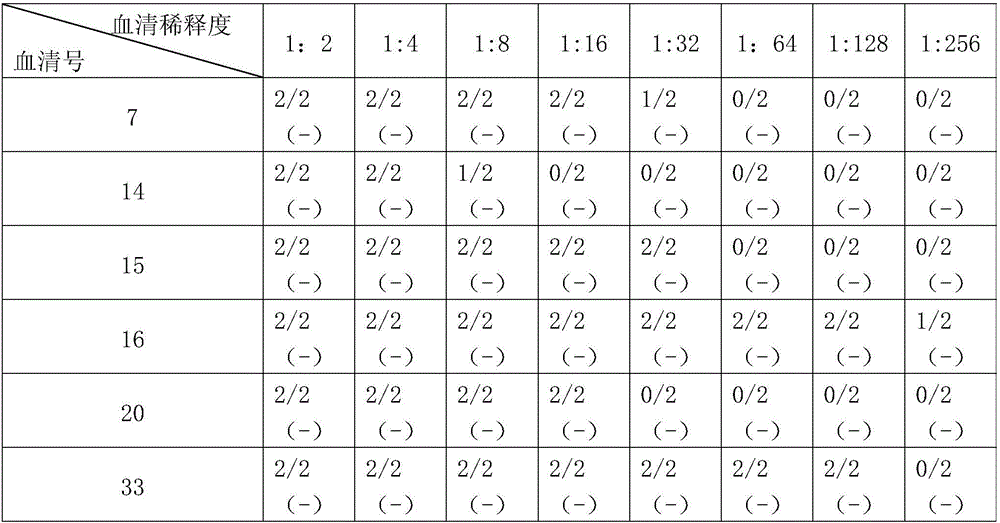
[0059] Embodiment 2, the quantitative detection of swine fever neutralizing antibody titer in pig serum
[0060] The quantitative detection method of swine fever neutralizing antibody titer in pig serum of the present invention comprises the following steps:
[0061] 1) Inactivation of the pig serum to be tested: inactivate the aseptically collected pig serum to be tested in a 56°C water bath for 30 minutes (55-57°C water bath inactivation for 30-40 minutes is acceptable);
[0062] 2) Porcine serum dilution: Add 50 μL of MEM culture solution to each well of the cell culture plate, then add 50 μL of the porcine serum to be tested in the first well and mix, take out 50 μL with a micropipette, add to the second well, mix Take out 50 μL after homogenization, and then add it to the third well, and so on, until the last well (discard 50 μL of the mixed solution), the dilution ratio of pig serum is 1:2, 1:4, 1:8, 1:16 , 1:32, 1:64, set 2 (2-4) parallel wells for each different dilut...
Embodiment 3
[0081] Example 3, Quantitative detection kit for neutralizing antibody titer to classical swine fever in pig serum
[0082] The quantitative detection kit of the neutralizing antibody titer of classical swine fever in the swine serum of the present invention comprises the anti-CSFV E2 Monoclional Antibody (primary antibody) of classical swine fever monoclonal antibody and the goat anti-mouse IgG (secondary antibody) labeled with peroxidase ).
[0083] For the convenience of detection, the kit of the present invention also includes 0.01mol PBS with pH 7.4, peroxidase substrate chromogenic solution, 80% acetone fixative solution and the like.
[0084] For the method of using the kit of the present invention, refer to Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com