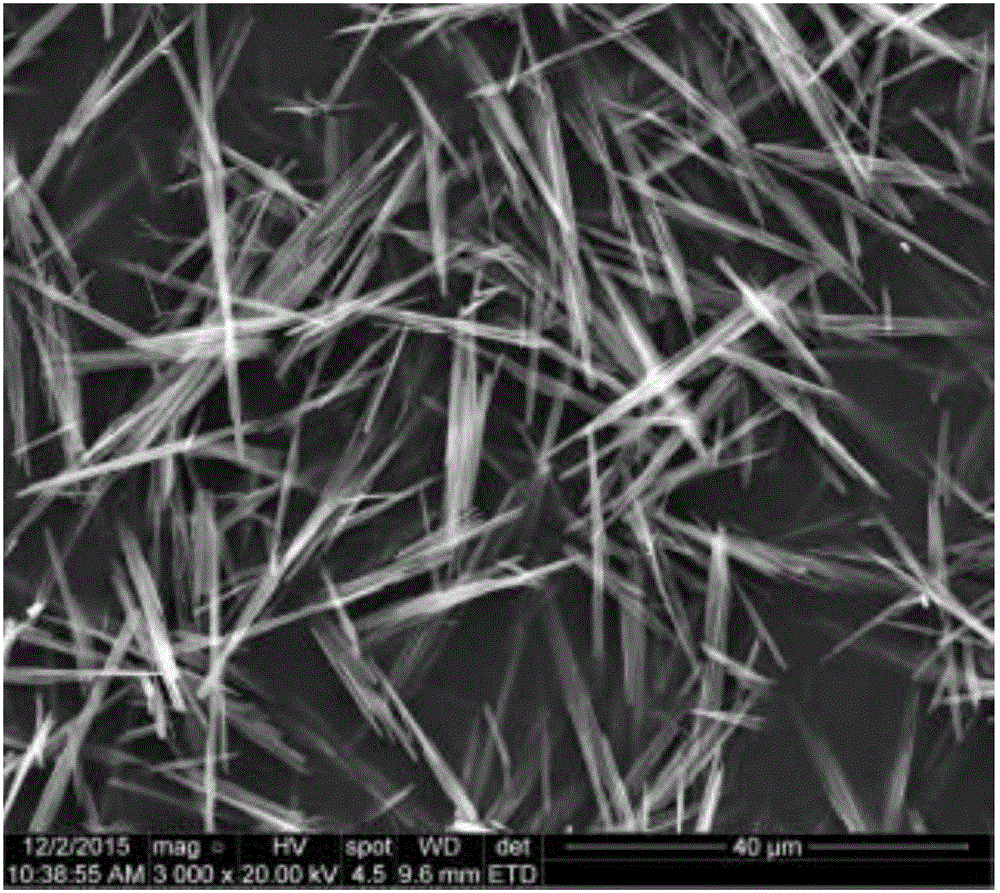
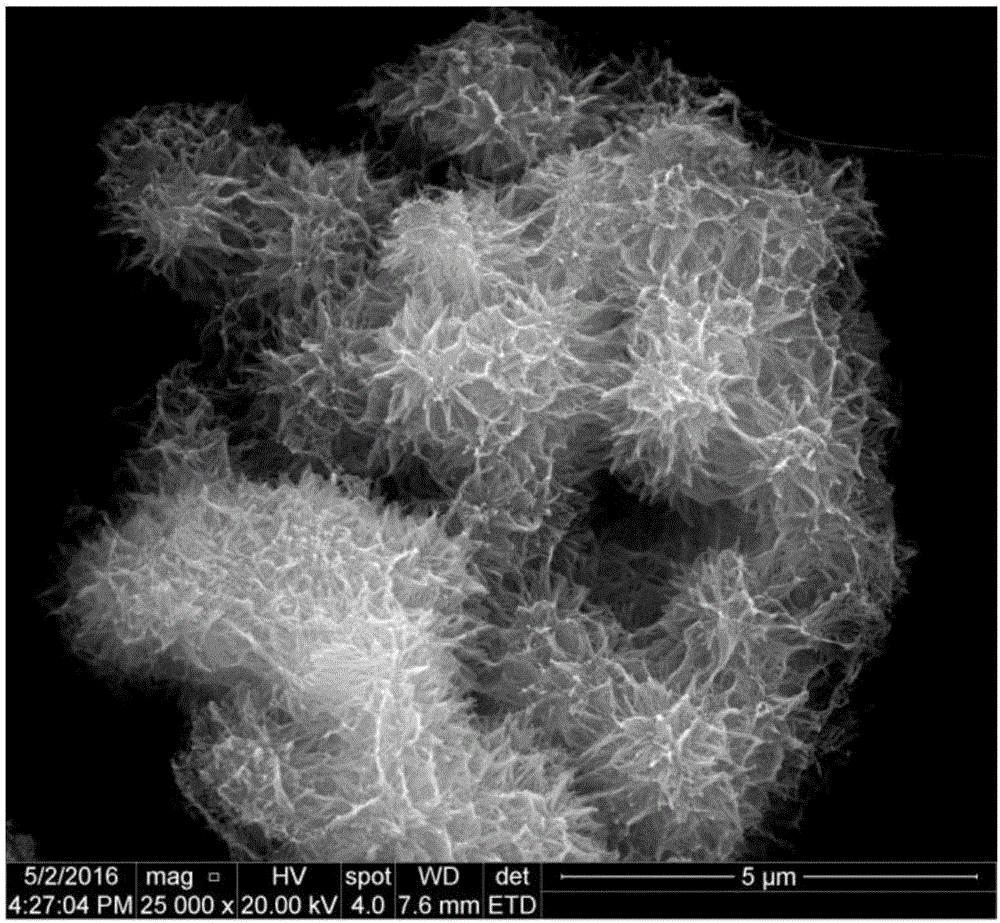
Preparation method of copper benzoate hydroxide-SiO2 composite material with photocatalysis
A technology of copper hydroxide benzoate and composite materials, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, special compound water treatment, etc., can solve the problem of low energy utilization rate of photocatalytic materials, dyes Due to the poor photocatalytic degradation effect of wastewater, the overall morphology is complete, the preparation method is simple and effective, and the degradation efficiency is improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Step 1, weigh 3.0g of copper nitrate and dissolve it in 50mL of water to obtain a copper nitrate solution, add 2.5mL of concentrated ammonia water dropwise to the copper nitrate solution, and keep stirring until a light blue precipitate is formed, then continue stirring at room temperature for 0.5h , after the solution was completely precipitated, aged for 6 hours, suction filtered, and the precipitate was washed several times with deionized water, and then dried at room temperature for 24 hours to obtain copper hydroxide;
[0036] Step 2: Put 0.7140g of copper hydroxide and 0.7170g of benzoic acid in 20mL of deionized water, and carry out a reflux reaction at 95°C for 24 hours under full stirring. After the reaction is completed, the product is suction filtered, washed, and dried at room temperature for 24 hours. That is, layered copper hydroxide benzoate (LDH-Cu).
[0037] Step 3, at room temperature, mix 31.5mL deionized water, 30mL ammonia water and 20mL absolute et...
Embodiment 2
[0040]Step 1, weigh 3.0 g of copper nitrate and dissolve it in 50 mL of water to obtain a copper nitrate solution, add 3 mL of concentrated ammonia water dropwise to the copper nitrate solution, and keep stirring until a light blue precipitate is formed, continue stirring for 1 h at room temperature, and wait for After the solution was completely precipitated, aged for 8 hours, suction filtered, and the precipitate was washed several times with deionized water, and then dried at room temperature for 32 hours to obtain copper hydroxide;
[0041] Step 2, put 1.5g of copper hydroxide and 1g of benzoic acid in 45mL of deionized water, and carry out a reflux reaction at 90°C for 18h under sufficient stirring. After the reaction is completed, the product is suction filtered, washed, and dried at room temperature for 32h, namely Layered copper hydroxide benzoate (LDH-Cu) was obtained.
[0042] Step 3, at room temperature, mix 30mL deionized water, 30mL ammonia water and 20mL absolute...
Embodiment 3
[0045] Step 1, weigh 3.0 g of copper nitrate and dissolve it in 50 mL of water to obtain a copper nitrate solution, add 2 mL of concentrated ammonia water dropwise to the copper nitrate solution, and keep stirring until a light blue precipitate is formed, continue stirring for 45 minutes at room temperature, and wait for After the solution was completely precipitated, aged for 7 hours, suction filtered, and the precipitate was washed several times with deionized water, and then dried at room temperature for 28 hours to obtain copper hydroxide;
[0046] Step 2, put 1.2g of copper hydroxide and 1g of benzoic acid in 36mL of deionized water, and carry out a reflux reaction at 100°C for 20h under sufficient stirring. After the reaction is completed, the product is suction filtered, washed, and dried at room temperature for 28h, namely Layered copper hydroxide benzoate (LDH-Cu) was obtained.
[0047] Step 3, at room temperature, mix 36mL deionized water, 30mL ammonia water and 24mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com