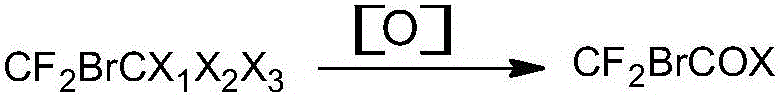
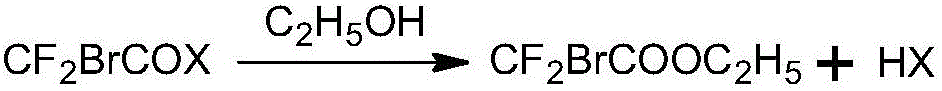Preparation method of ethyl bromodifluoroacetate
A technology of ethyl difluorobromoacetate and difluorobromoacetonitrile, which is applied in the field of preparation of ethyl difluorobromoacetate, can solve the problems of poor selectivity, easy hydrolysis, damage, etc., and achieve the effect of reducing requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of difluorobromoacetonitrile from difluorodibromomethane
[0041]
[0042]Add 90 g of cuprous cyanide powder to 500 ml of 1:1 (volume ratio) N,N-dimethylformamide (DMF) / ethylene glycol dimethyl ether (DME) mixed solvent, and add 1 g of catalyst Dibenzo-18-crown-6, slowly add a molar amount of difluorodibromomethane dissolved in DMF / DME (volume ratio 1:1) mixed solution, and start monitoring after half an hour of reaction at 20°C , monitored by GC (column: PEG-20M) until the conversion was 80%.
[0043] The temperature was slowly raised to 50°C to evaporate the difluorobromoacetonitrile, and then the difluorobromoacetonitrile was collected at a low temperature through a cold well below 0°C.
[0044] Carbon NMR spectrum: 13 CNMR: σ C 115.5ppm, σ C 98.6ppm.
Embodiment 2
[0046] Add 90 g of cuprous cyanide powder to 500 ml of 1:2 (volume ratio) N,N-dimethylformamide (DMF) / ethylene glycol dimethyl ether (DME) mixed solvent, and add 2 g of catalyst Dibenzo-18-crown-6 was slowly added dropwise to a mixed solution of difluorodibromomethane dissolved in DMF / DME (volume ratio 1:2). Column: PEG-20M) The reaction was stopped when the conversion rate was 85%. Slowly raise the temperature to 50°C to evaporate the difluorobromoacetonitrile, and then pass through a cold well below 0°C to collect the difluorobromoacetonitrile at a low temperature.
[0047] Carbon NMR spectrum: 13 CNMR: σ C 115.5ppm, σ C 98.4ppm.
Embodiment 3
[0049] Add 90 g of cuprous cyanide powder to 500 ml of 1:3 (volume ratio) N,N-dimethylformamide (DMF) / ethylene glycol dimethyl ether (DME) mixed solvent, and add 3 g of catalyst Dibenzo-18-crown-6 was slowly added dropwise to the mixed solution of difluorodibromomethane DMF / DME, and the reaction was carried out at 20°C for half an hour, and the conversion rate was measured by GC (chromatographic column: PEG-20M). up to 90%. Slowly raise the temperature to 50°C to evaporate the difluorobromoacetonitrile, and then collect the difluorobromoacetonitrile at a low temperature through a cold well below 0°C.
[0050] Carbon NMR spectrum: 13 CNMR: σ C 115.4ppm,σ C 98.6ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com