Novel labdane-type diterpenoid compound, preparation method and application thereof, pharmaceutical composition and application of pharmaceutical composition
A kind of compound, the technology of haloane, which is applied in the field of helianthane type diterpenoids and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
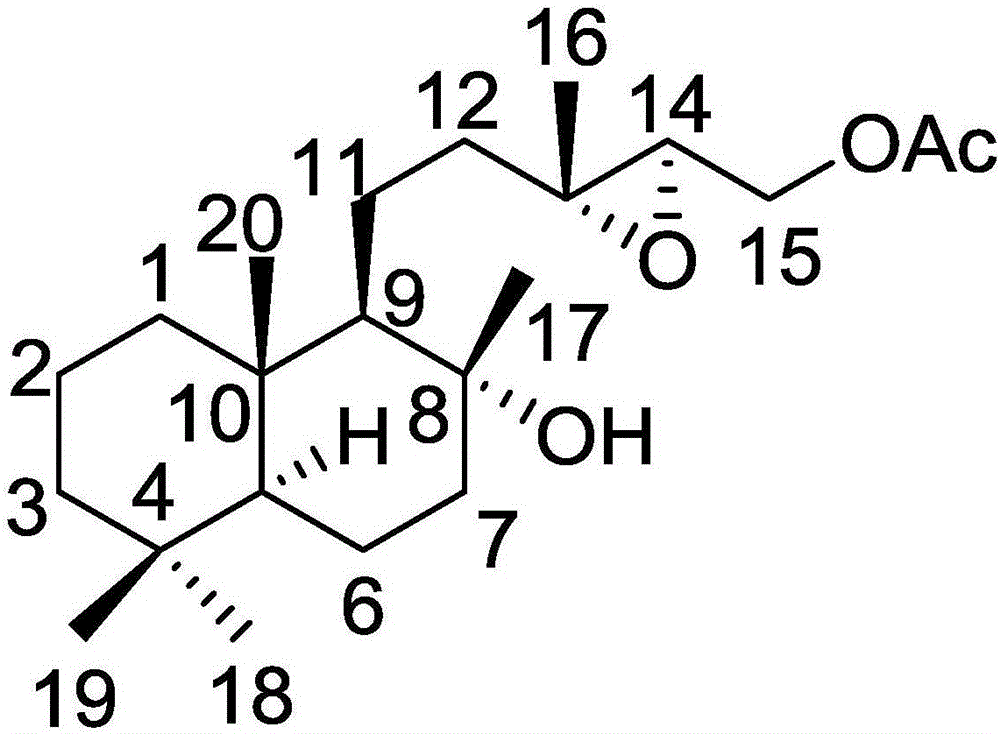
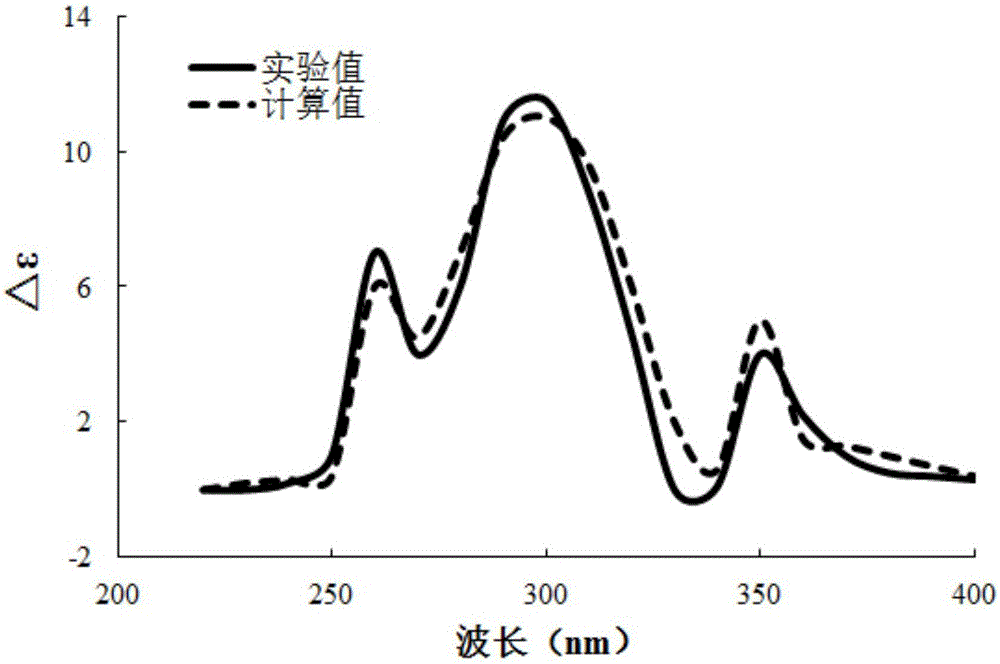
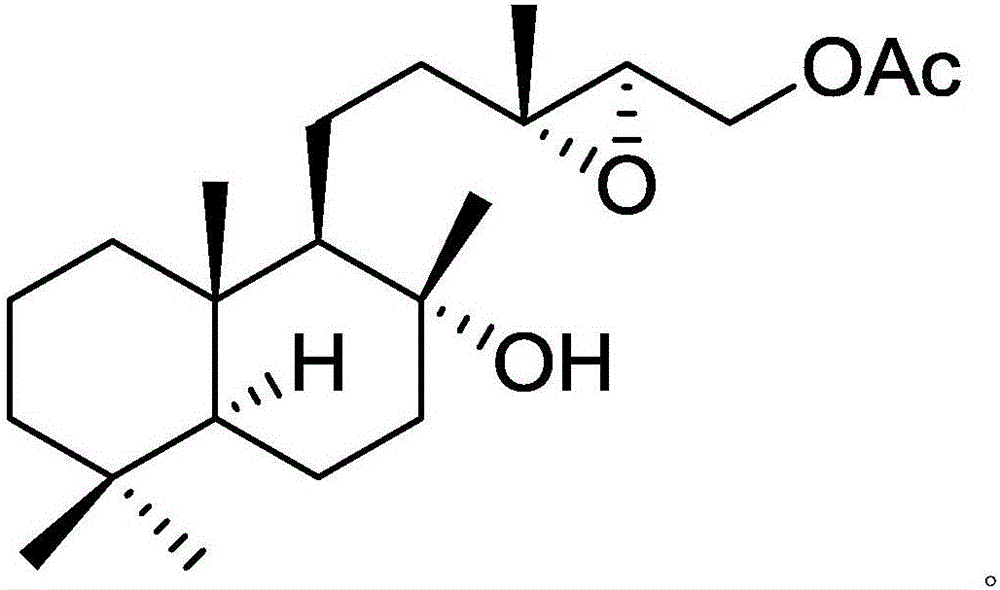
[0022] Example 1: Isolation, Preparation and Structure Confirmation of a New Helicanoid Diterpene Compound
[0023] Sources of reagents: ethanol, petroleum ether, ethyl acetate, n-butanol, and dichloromethane were of analytical grade, purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. Methanol, of analytical grade, were purchased from Jiangsu Hanbang Chemical Reagent Co., Ltd.
[0024] Preparation method: (a) Grind the dry aerial part (10kg) of Aurora nudiflora, extract with 75% ethanol under hot reflux (25L×3 times), combine the extracts, concentrate until no alcohol smell (3L), and successively use petroleum Ether (3L×3 times), ethyl acetate (3L×3 times) and water-saturated n-butanol (3L×3 times) were extracted to obtain petroleum ether extract, ethyl acetate extract (431g) and n-butanol Extract; (b) in step (a), the ethyl acetate extract is removed with D101 macroporous resin, first eluted with 10% ethanol for 8 column volumes, then with 80% ethanol for 10 column v...
Embodiment 2
[0026] Embodiment 2: A kind of pharmacological effect test of new helianthene type diterpene compound
[0027] 1. Materials and Instruments
[0028] Human pancreatic cancer MiaPaCa-2 cells were kindly donated by the Cancer Research Institute of the Cancer Hospital Affiliated to Tianjin Medical University. The compound is self-made, and the HPLC normalized purity is greater than 98%. FBS and trypsin-EDTA digestion solution were purchased from Hyclone Company in the United States. PBS powder was purchased from Tianjin Runtai Technology Development Co., Ltd. DMEM low-sugar medium was purchased from Gibco, USA. MTT was purchased from Sigam, USA. DMSO was purchased from Beijing Chemical Plant. Penicillin Sodium for Injection was purchased from Harbin Pharmaceutical Group General Pharmaceutical Factory. Streptomycin sulfate for injection was purchased from Dalian Merro Pharmaceutical Factory.
[0029] Ultra-clean bench, cell incubator (Thermo Company, USA), 4°C refrigerator, ...
Embodiment 3
[0053] Preparation of tablets: firstly prepare the compound according to the method in Example 1, and utilize organic acids such as tartaric acid, or citric acid or formic acid or oxalic acid, etc., inorganic acids such as hydrochloric acid or sulfuric acid or phosphoric acid to make salts; Add excipients at a weight ratio of 1:7, granulate and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com