Development method of fluorescent protein transgenic zebra fish capable of highly sensitively monitoring water body PAHs by utilizing cascade amplification effect
A fluorescent protein and zebrafish technology, applied in the biological field, can solve problems such as the inability to transmit LUC reporter genes and the failure to monitor aromatic hydrocarbon pollutants, and achieve high sensitivity results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
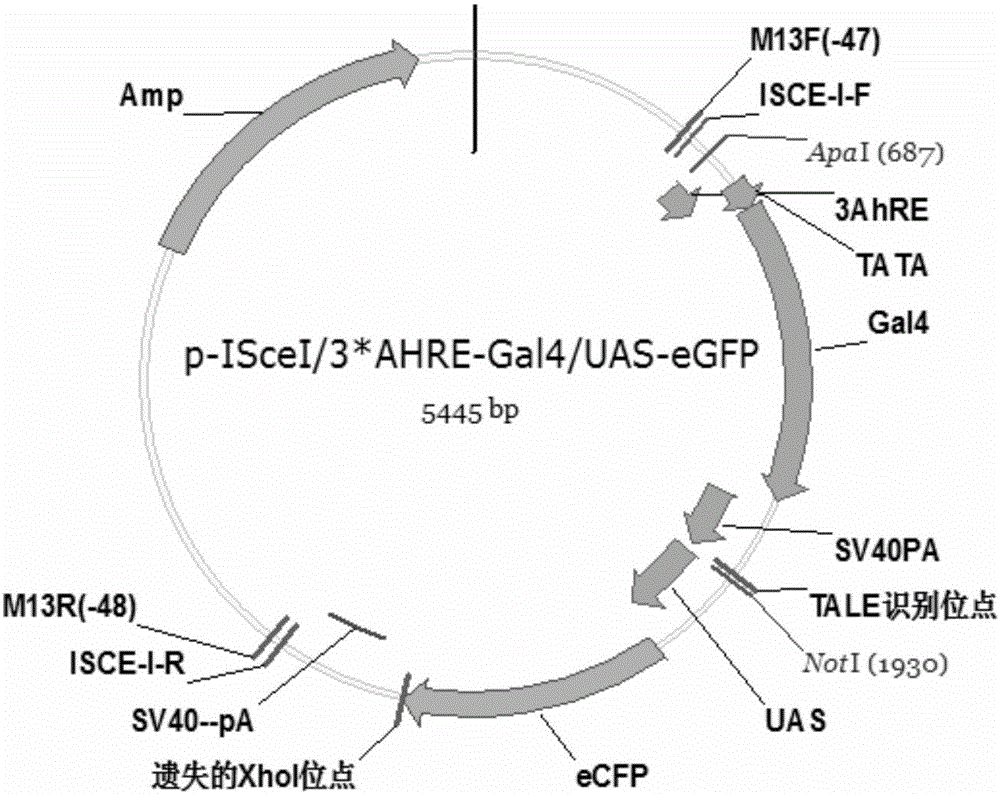
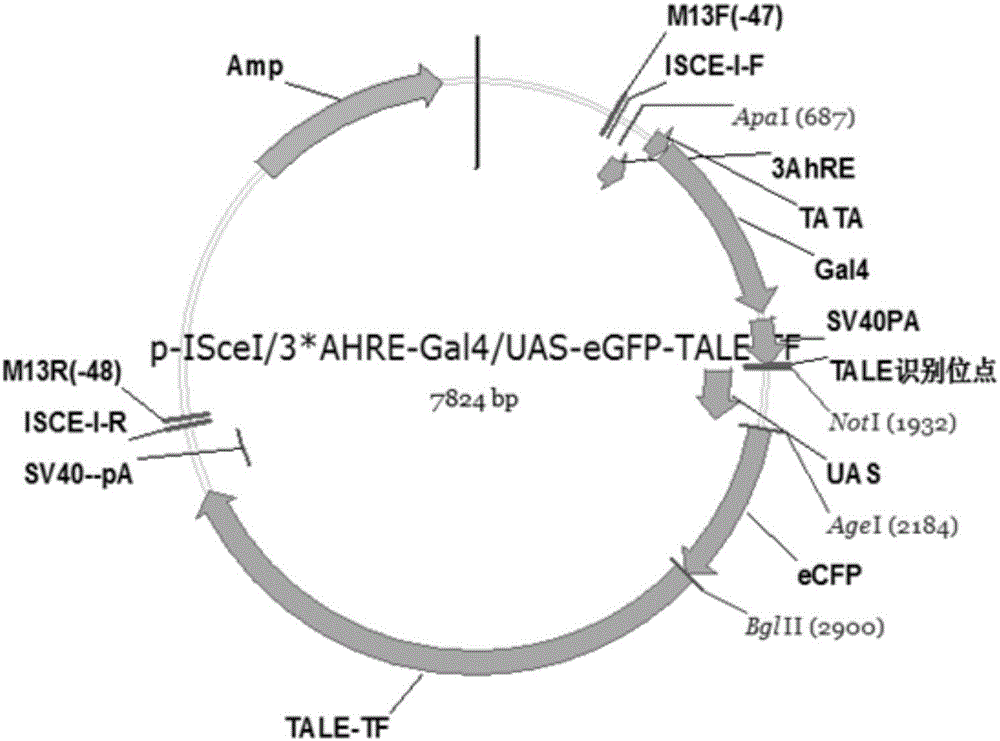
[0035] 1. Construction of GAL4-UAS upstream response carrier capable of responding to polycyclic aromatic hydrocarbons in water environment
[0036] 1. Synthesis of polycyclic aromatic hydrocarbon response element AhREs:
[0037] A mutant of the AhREs sequence: TCCGGTCCTTCTCACGCAACGCCTGGG (SEQ ID NO.1) was selected as the basic element.
[0038] Artificially synthesized gene fragment NotI-NheI-AhREs-SpeI-M.EcoP15I-NcoI-TATA+-PstI with 3 copies in tandem in the same direction, including the corresponding restriction site and the downstream part of the TATA-box sequence:
[0039] 5'- GCGGCCGCGCTAGC TCCGGTCCTTCTCACGCAACGCCTGGGTCCGGTCCTTCTCACGCAACGCCTGGGTCCGGTCCTTCTCACGCAACGCCTGGG ACTAGTCAGCAGCCATGG TTCGCATATTAAGGTGACGCGTGTGGCCTCGAACACCGAGCGACC CTGCAG -3' (SEQ ID NO.2) (the underlined part is the restriction site, the bold is the 3-fold tandem AhREs mutant sequence, and the italic is the partial sequence of AhREs downstream TATA-box).
[0040] The artificially synthesized f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com