Method for enzymatic synthesis of 1,2-diacylglycerol and method for purifying obtained 1,2-diacylglycerol
A technology for diglyceride and enzymatic synthesis, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as poor thermal stability, and achieve the effect of reducing difficulty, mild conditions, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
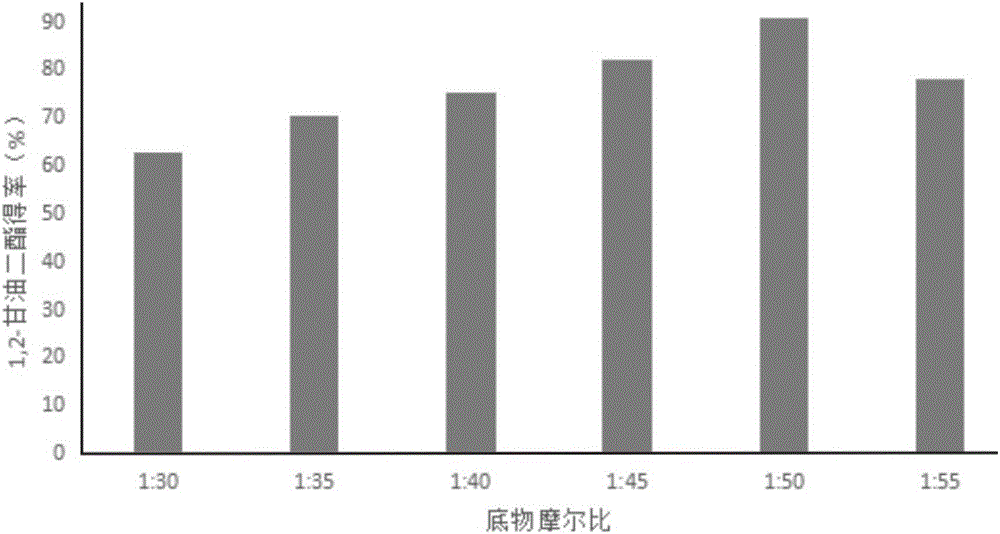
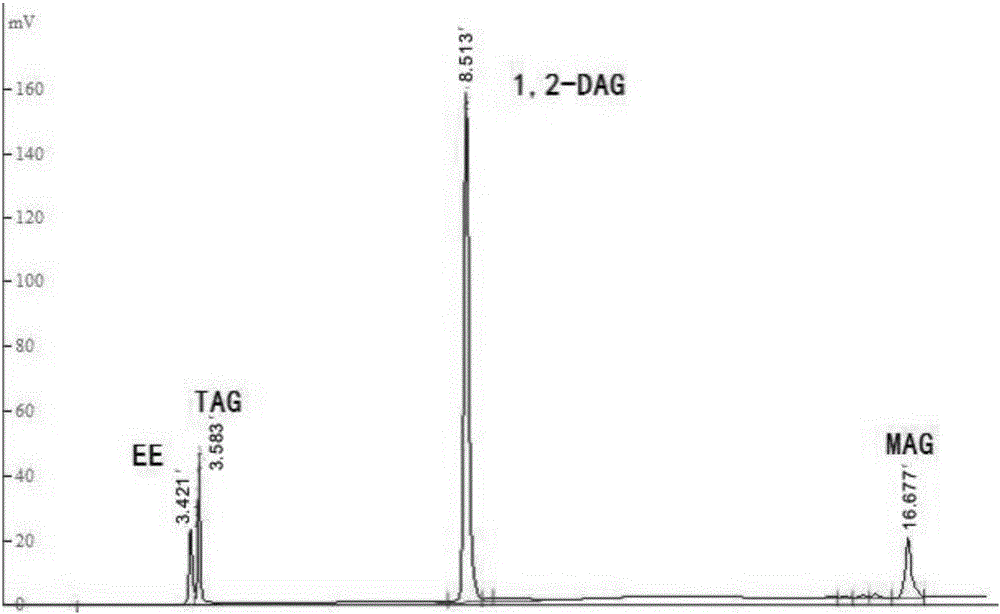
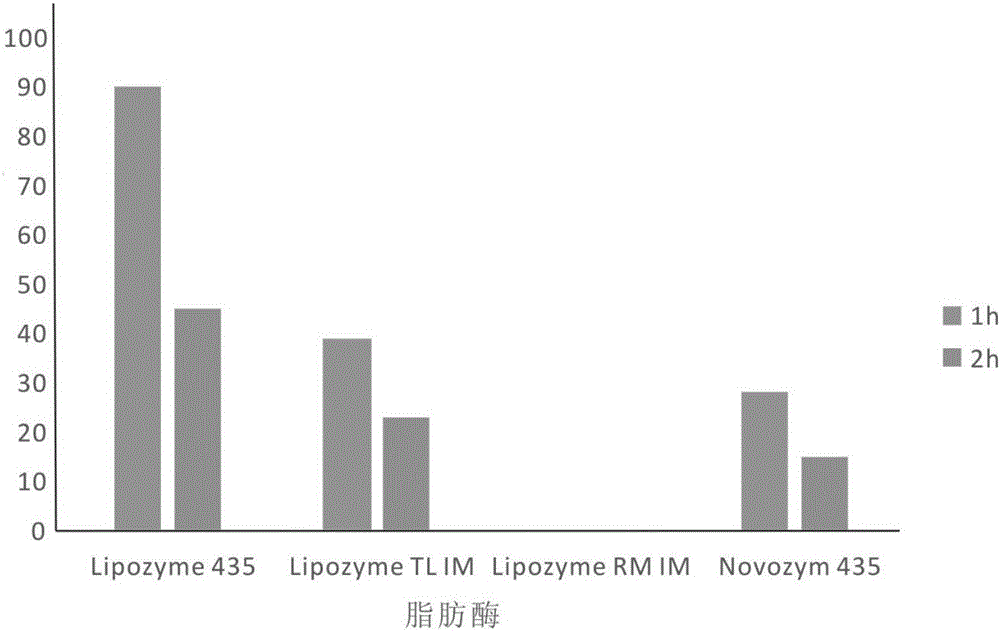
[0040] Add high oleic sunflower oil and n-propanol into the reaction kettle at a molar ratio of 1:30, put a stirring bar, preheat in a constant temperature water bath at a temperature of 40°C, turn on the magnetic stirrer, and stir at a speed of 500rpm After emulsifying for 15 minutes, add lipase Lipozyme RM IM, which accounts for 6% of the total mass of the substrate, to start the reaction. After 1 hour of reaction, the product is taken out, and the lipase is removed by centrifugation at 4000 rpm for 10 minutes. After HPLC analysis, the content of 1,2-diglyceride in the product is The yield was 45.3%.
Embodiment 2
[0042] Add high oleic sunflower oil and absolute ethanol into the reaction kettle at a molar ratio of 1:50, put a stirring bar, preheat it in a constant temperature water bath at a temperature of 50°C, turn on the magnetic stirrer, and set it at a speed of 500rpm After stirring and emulsifying for 15 minutes, add lipase Lipozyme 435 accounting for 6% of the total mass of the substrate to start the reaction. After 1 hour of reaction, the product is taken out and centrifuged at 4000 rpm for 10 minutes to remove the lipase. After HPLC analysis, the content of 1,2-diglyceride in the product is The yield was 90%.
Embodiment 3
[0044] Add palm oil and absolute ethanol to the reaction kettle at a molar ratio of 1:40, put a stirring bar, preheat in a constant temperature water bath at a temperature of 45°C, turn on the magnetic stirrer, stir and emulsify at a speed of 500rpm for 15min , add lipase Lipozyme 435 accounting for 8% of the total mass of the substrate to start the reaction. After 1 hour of reaction, the product is taken out and centrifuged at 4000prm for 10 minutes to remove the lipase. According to HPLC analysis, the yield of 1,2-diglyceride in the product is 80.5 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com