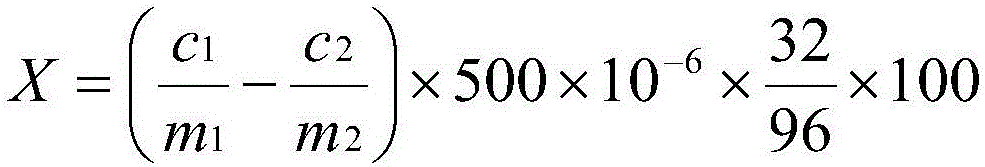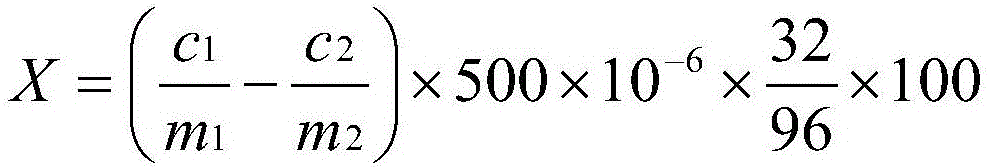Method for determining elemental sulfur in fertilizer
A determination method and technology of elemental sulfur, applied in the field of phosphorus chemical industry, can solve the problems of large limitations, hidden dangers of production safety cannot be effectively monitored and monitored, and time-consuming, etc., to achieve improved accuracy and precision, and long-term monitoring of hidden dangers of safety. , sensitive and selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 1. Instruments: electronic balance, Prodigy XP inductively coupled plasma emission spectrometer (Leaman Corporation, USA)
[0062] 2. Reagents: potassium chlorate (analytical pure), concentrated nitric acid (analytical pure), (1+1) nitric acid, SO 4 Standard stock solution (1mg / mL)
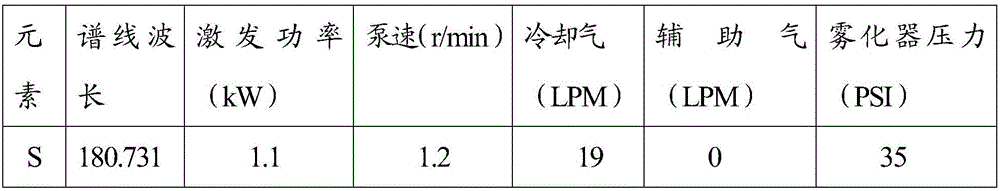
[0063] 3 Analytical conditions: the parameter settings of the atomic emission spectrometer are shown in Table 1
[0064] Table 1 Test parameters of atomic emission spectrometer
[0065]
[0066] 4. Establish a working curve:
[0067] Take 0.00mg, 50mg, and 100mg standard stock solutions respectively and put them in a series of 500mL volumetric flasks, dilute to the mark with pure water, shake well, and detect the sulfate radical value on an atomic emission spectrometer. Take the quality mg of sulfate radical as the abscissa, and the corresponding atomic emission spectrometer value as the ordinate, draw the working curve with the detection value of the sulfate radical standard solution...
Embodiment 2
[0085] Precision experiment
[0086] Weigh 0.4012g and 0.4008g samples respectively and measure 10 sets of data according to the test method in Example 1, and the test results are shown in Table 3.
[0087] Table 3 precision measurement data
[0088] serial number 1 2 3 4 5 6 7 8 9 10 RSD% Total Sulfur % 7.25 7.43 7.55 7.33 7.38 7.50 7.38 7.43 7.46 7.40 1.15 Compound sulfur% 3.80 3.99 3.90 3.86 3.89 3.84 3.82 3.86 3.87 3.92 1.40 Elemental sulfur 3.45 3.44 3.65 3.47 3.49 3.66 3.56 3.57 3.59 3.48 0.02
[0089] It can be seen from Table 3 that the extreme difference of the results of 10 groups is less than or equal to 0.2%, the RSD is less than 2%, and the interference of the matrix is small, indicating that the precision of the present invention is better.
Embodiment 3
[0091] Accuracy experiment
[0092] Three groups of samples were weighed, and a standard addition recovery experiment was carried out to measure the accuracy of the detection method of the present invention. The experimental data are shown in Table 4.
[0093] Table 4 horizontal atomic emission spectrometer plus diammonium thiophosphate standard recovery test data
[0094]
[0095]
[0096] It can be seen from Table 4 that the recovery rates of the samples were all above 96.8%, indicating that the detection method of the present invention has better accuracy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com