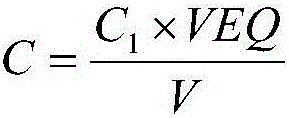Method for measuring content of chlorine in mixed acid electrolyte through potentiometric titration
A potentiometric titration and electrolyte technology, which is used in chemical analysis, measuring device, chemical method analysis, etc. by titration method, can solve the problem that the content of trivalent and tetravalent vanadium cannot be determined, it is not suitable for vanadium ion solution, and cannot be accurately measured. Solve problems such as solution state, achieve accurate determination of valence state and total vanadium content, improve stability and accuracy, and smooth the oxidation titration curve.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1. Preparation of silver nitrate standard solution
[0059] 1A. Weigh 8.5g of commercially available analytically pure silver nitrate and dissolve it in 1000mL of high-purity water, and transfer it to a brown reagent bottle for storage until calibration;
[0060] 2. Calibration of silver nitrate standard solution
[0061]2A. Weigh 0.9g (accurate to 0.0001g) of standard sodium chloride and burn it at 500-600°C to constant weight, put it in a 250mL quartz beaker, add high-purity water to dissolve;
[0062] 2B. Quantitatively transfer the solution in 2A into a 250mL volumetric flask, make up to volume with high-purity water, shake well, transfer to a thin-mouthed polyethylene bottle for storage, label the reagent well, and store it at room temperature in the dark;
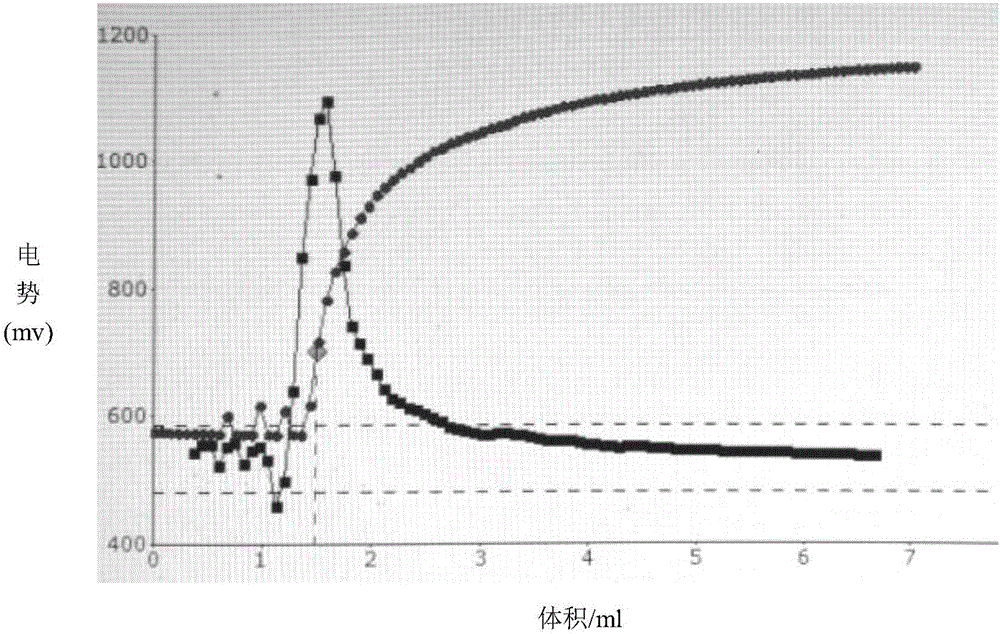
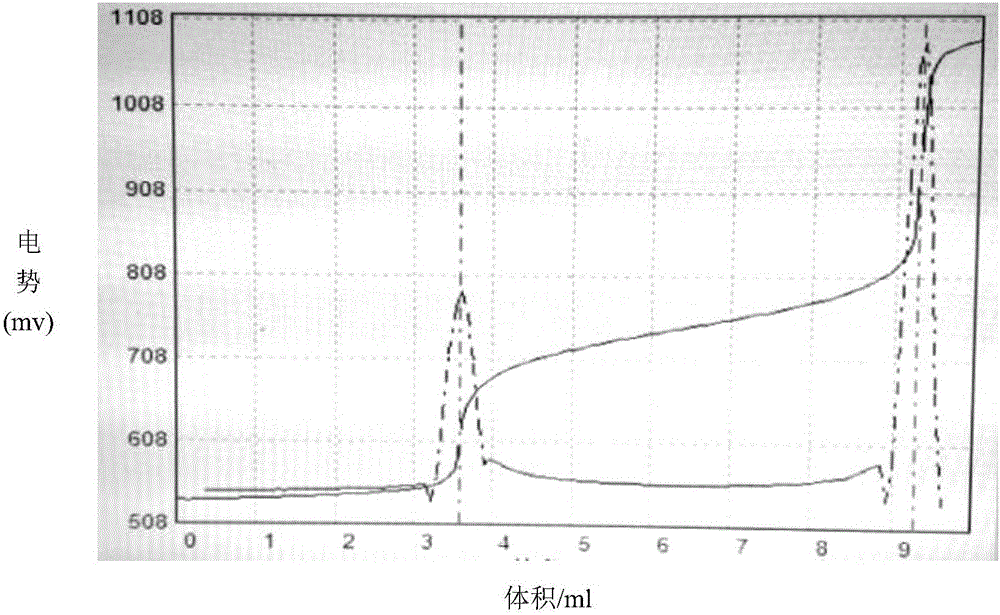
[0063] 2C. Accurately pipette 5.00mL of the sodium chloride standard solution in 2B into a plastic beaker, add 40mL of high-purity water and 1mL of nitric acid (1+1) in sequence, and titrate to the end point w...
experiment example 2
[0088] ---Precision, accuracy, and recovery experiments of the chloride ion detection method of the present invention
[0089] (1) Precision experiment
[0090] Experimental method: select 5 different electrolyte samples, respectively measure the chloride ion concentration according to the detection method described in Example 1, and calculate the relative standard deviation. The experimental results are shown in Table 1.
[0091] The precision experiment result of the detection method of the present invention in table 1
[0092] sample Measured value (mol / L) Average(mol / L) Relative standard deviation RSD Electrolyte sample 1 7.827,7.817,7.821,7.815,7.826 7.821 0.07 Electrolyte sample 2 8.537,8.523,8.539,8.515,8.532 8.529 0.12 Electrolyte sample 3 8.668,8.675,8.672,8.673,8.661 8.670 0.06 Electrolyte sample 4 9.685,9.717,9.684,9.699,9.705 9.698 0.14 Electrolyte sample 5 7.776,7.785,7.798,7.794,7.786 7.788 0.11
[0093...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com