Method for preparing diaryl ketone by means of spontaneously oxidizing diaryl alkane under promotion effect of alkali
A technology of diaryl alkanes and methoxy groups, which is applied in the field of alkali-promoted autoxidation of diaryl alkanes to prepare diaryl ketones, which can solve the problems of poor recyclability, by-products, and low catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
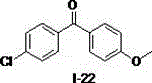
[0015] Example 1, using diphenylmethane to prepare diphenyl ketone as an example to illustrate the reaction operation and detect the influence of different solvents on the autoxidation reaction (taking the I-1 compound shown in the formula as an example)
[0016] Diphenylmethane (0.2 mmol), different reaction solvents (0.5 mL) (dimethylsulfoxide, N,N-dimethylformamide, tetrahydrofuran, ethyl acetate, acetonitrile, dichloromethane) and sodium tert-butoxide (0.4 mmol) into the reaction flask in turn, hang an oxygen balloon at 50 o C was reacted for 0.5 hours. Add water, dilute hydrochloric acid successively to the reaction solution, extract with ethyl acetate, and separate the oxidation product by silica gel column chromatography. The calculated separation yield is shown in Table 1, wherein, the yield of the target product ketone in dimethyl sulfoxide The highest value was obtained, which was 92%, and the best solvent was determined as dimethyl sulfoxide.
[0017]
[0018] ...
Embodiment 2
[0022] Embodiment 2, the influence of temperature of reaction on autooxidation reaction of the present invention
[0023] In addition to the different reaction temperatures (25 o C. 30 o C. 40 o C, 50 o C. 60 o C. 70 o C. 80 o C), other reaction conditions were the same as in Example 1, and the influence of reaction temperature on oxidation reaction yield was detected. After the reaction finishes, the separation yield measurement result of target ketone is as shown in table 2, shows that along with the change of reaction temperature, there is an optimum value in the yield of oxidation reaction, and optimum reaction temperature is determined as 50 oC .
[0024] temperature reflex( o c)
Embodiment 3
[0025] Embodiment 3, the influence of different bases on the autoxidation reaction of the present invention
[0026] Except that the reaction base is different (sodium tert-butoxide, potassium tert-butoxide, sodium hydride, potassium hydroxide, sodium hydroxide, potassium carbonate, DBU), other reaction conditions are the same as in Example 1. Yield impact. After the reaction finishes, the separation yield measurement result of target ketone is as shown in table 3, shows that stronger base can all promote oxidation reaction, and tert-butanol base and hydroxide base are the best among the bases tested, especially tert-butanol Sodium is optimal.
[0027] alkali Sodium tert-butoxide Potassium tert-butoxide sodium hydride Potassium hydroxide sodium hydroxide Potassium Carbonate or DBU Separation yield (%) 92 84 77 79 80 N.R.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com