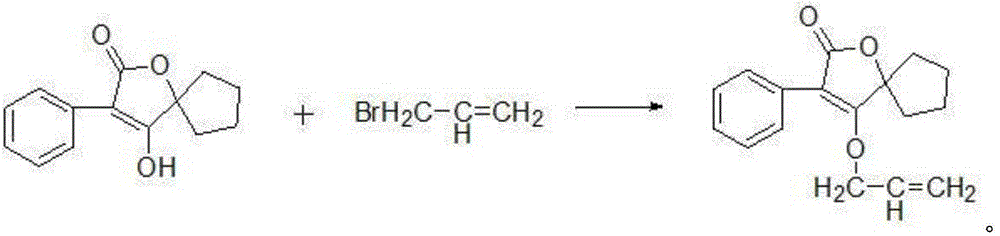Volution lactone compound and synthesis method and application thereof
A technology of ester compounds and compounds, which is applied in the field of pesticides and achieves the effects of long lasting effect, good acaricidal effect and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
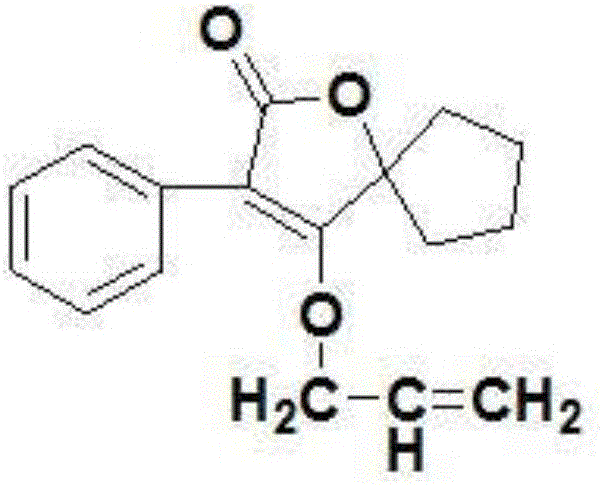
[0030] Embodiment one: a kind of spirolide compound provided by the present invention, its chemical structural formula is:
[0031]
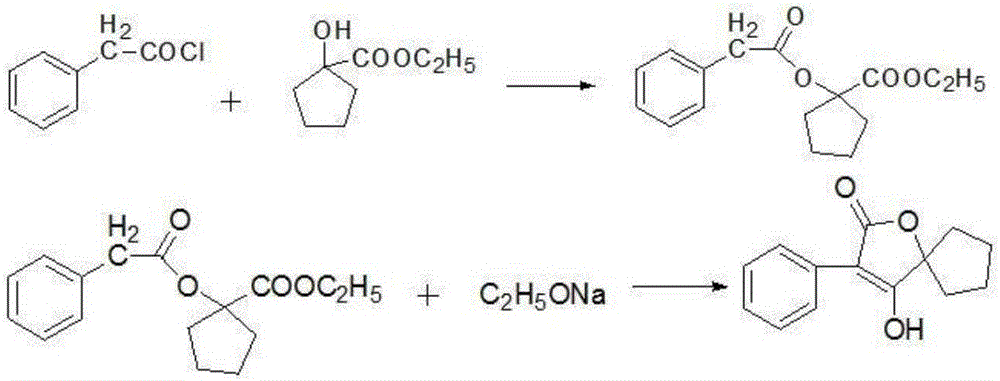
[0032] The method for preparing the above-mentioned compound comprises the following steps, (1) using cyclopentanone as a raw material, after carrying out cyanohydrinization reaction, hydrolyzing and esterifying the cyano group to obtain ethyl hydroxycyclopentyl carboxylate;
[0033] (2) sulfonation reaction: synthesis of phenylacetyl chloride;
[0034] (3) Docking and cyclization reaction: the ethyl hydroxycyclopentyl carboxylate obtained in step (1), the phenylacetyl chloride and ethanol obtained in step (2) are mixed, after heating up and stirring, slowly add ethanol with a mass concentration of 20% Sodium ethanol solution, the ethanol is distilled off under reduced pressure after the reaction, and then hydrochloric acid with a mass concentration of 20% is added dropwise to obtain 4-hydroxy-3-phenyl-1-oxaspiro[4.4]non-3-ene after filtratio...
Embodiment 2
[0040] Embodiment two: a kind of spirolide compound provided by the present invention, its chemical structural formula is:
[0041]
[0042] The method for preparing the above-mentioned compound comprises the following steps, (1) using cyclopentanone as a raw material, after carrying out cyanohydrinization reaction, hydrolyzing and esterifying the cyano group to obtain ethyl hydroxycyclopentyl carboxylate;
[0043] When selecting the above-mentioned synthetic route, the applicant of the present invention weighs according to the recovery rate and the cost, comprehensively finally selects the synthetic route disclosed by the present invention, and enumerates the test method for selecting the synthetic route below, in order to illustrate that the synthetic route finally selected by the present invention is the most Excellent choice:
[0044] 1. Synthesis of ethyl hydroxycyclopentyl carboxylate
[0045] 1.1 Ethyl hydroxycyclopentyl carboxylate is obtained by hydrolyzing and es...
Embodiment 3
[0101] Embodiment three: a kind of spirolide compound provided by the present invention, its chemical structural formula is:
[0102]
[0103] The method for preparing the above-mentioned compound comprises the following steps, (1) using cyclopentanone as a raw material, after carrying out cyanohydrinization reaction, hydrolyzing and esterifying the cyano group to obtain ethyl hydroxycyclopentyl formate: cyclopentanol, catalyst sodium pyrosulfate, The water is stirred and dissolved, the molar ratio of cyclopentanone and sodium cyanide is 1:0.95, the quality of the catalyst sodium pyrosulfate accounts for 0.3% of the reaction system quality, the reaction temperature is 30°C, and sodium cyanide is added dropwise in the mixed solution Aqueous solution, stirring, layering, the upper organic phase that obtains is cyclopentanyl cyanohydrin, the quality of water used can be as the criterion that can dissolve raw material,
[0104] The chemical reaction equation is:
[0105]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com