E-cadherin, Cadherin-11 and EpCAM multi-antibody immunomagnetic bead and preparation method thereof
An immunomagnetic bead and antibody technology, applied in the preparation of microspheres, chemical instruments and methods, magnetic materials, etc., can solve the problems of high cost and slow magnetic response, and achieve the effect of low cost, rapid magnetic response and good sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of Multiplex Antibody Immunomagnetic Beads
[0033] (1) Preparation of magnetic nanoclusters:
[0034] a. In the air, 7g FeCl 2 4H 2 O was added to 50 mL of deionized water to obtain a concentration of 0.14 g / mL of FeCl 2 aqueous solution. to 50mL FeCl 2 Add 30 mL of ammonia water to the aqueous solution, and after stirring for 45 minutes, the color gradually turns light green, then dark green, and finally black;
[0035] b. Add 1.1 g of oleic acid to step a, mix well, place the mixed solution in a closed reaction kettle, heat and react at 110°C for 4 hours, then alternately wash each time with deionized water and ethanol, after magnetic separation Dispersed in n-hexane, you can get black magnetic nano-cluster Fe 3 o 4 .
[0036] (2) Preparation of amino-modified magnetic microspheres: to 10 mg magnetic nanocluster Fe 3 o 4 1 solution, add 125 mg ammonia water, 30 mg tetraethyl orthosilicate and 30 mg (3-aminopropyl) triethoxysilane, rea...
Embodiment 2
[0043] Example 2 Sensitivity Detection of Multiple Antibody Immunomagnetic Beads
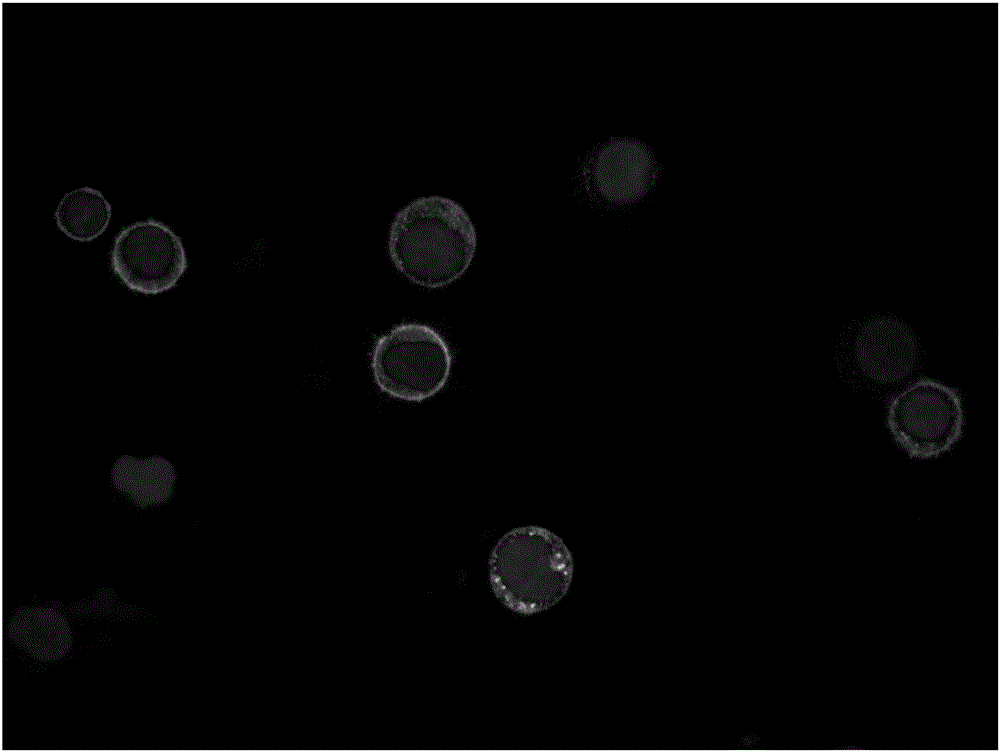
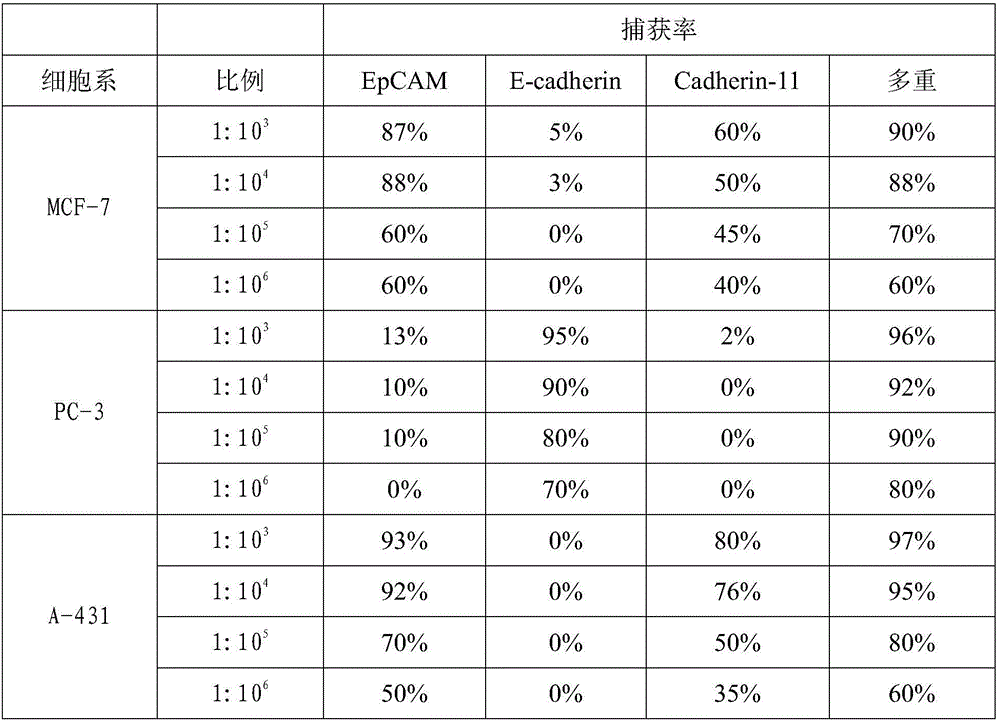
[0044] Human breast cancer cells MCF-7, human prostate cancer cells PC-3, and human epidermal cancer cells A-431 (all purchased from the Cell Bank of the Chinese Academy of Sciences) were taken, and the tumor cells in each group were divided into groups at a ratio of 1:10. 3 , 1:10 4 , 1:10 5 , 1:10 6 The ratio was added to PBMCs (5×10 6 )middle. Individual antibody immunomagnetic beads and multiple antibody immunomagnetic beads were added to the above-mentioned mixed cell suspensions, and incubated at 4°C for 30 minutes. Magnetic sorting was performed within 2 minutes and washed 2-3 times with PBS to obtain tumor cells captured and recovered with immunomagnetic beads. The proportion of recovered tumor cells to the total number of added tumor cells was counted, and the capture rate of immunomagnetic beads was calculated. The results are shown in Table 1:
[0045]
[0046] Table 1 Captur...
Embodiment 3
[0048] Example 3 Capture of Tumor Cells in Simulated Blood
[0049] Collect blood samples from healthy volunteers, mix PC-3 cells with peripheral blood of healthy people to make a mixed cell suspension, adjust the concentration of PC-3 cells to 1, 10, 20, 50, 500, 1000 cells / mL, and then Add the multiple antibody immunomagnetic beads to each of the above mixed cell suspensions in turn, and incubate at 4°C for 30 minutes. Magnetic sorting was performed within 1 minute and washed 2-3 times with PBS to obtain PC-3 cells captured and recovered with immunomagnetic beads. The ratio of recovered PC-3 cells to the total number of cells before capture was counted, and the capture efficiency of PC-3 cells in the blood sample captured by immunomagnetic beads was calculated, which was similar to the result in Example 2.
[0050] From the capture results of Examples 2-3, the capture efficiency of multiple antibody immunomagnetic beads in a simple environment (Example 2) can be accurate to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com