Synthesis of imidazole latent epoxy curing accelerator having toughening effect and application of accelerator in epoxy modification
A curing accelerator and epoxy modification technology, which is applied in the field of imidazole latent curing accelerators, can solve problems such as the lack of research on macromolecular imidazole latent curing accelerators, and achieve mass production and industrial applications. Good compatibility, the effect of low addition amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
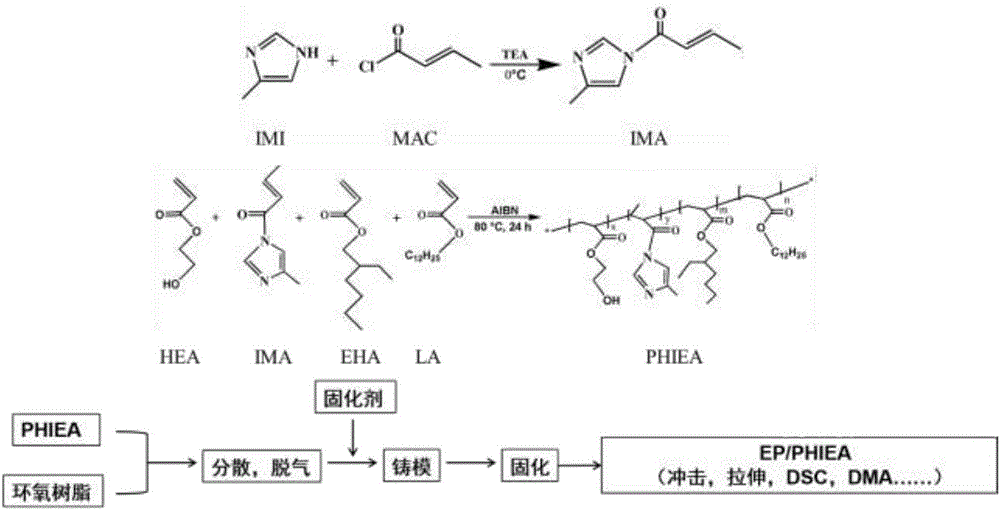
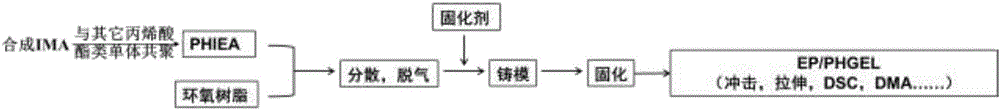
[0020] Step 1: Weigh 5.227g of methacryloyl chloride, dissolve it in 50mL of tetrahydrofuran (THF), and add it dropwise to 2-methylimidazole (4.105g) and triethylamine (6.07 g) in 100mL THF solution, stirred, and the above-mentioned process was carried out in a 250mL three-necked flask. After the dropwise addition, the stirring was continued at room temperature for 24-48 hours, and the reaction ended. The ammonium salt generated during the reaction is removed by suction filtration of the system, and the solvent is removed by rotary evaporation to obtain a free radical comonomer with an imidazole ring.
[0021] The second step: Weigh 3.380g of the monomer containing imidazole ring and double bond synthesized in the first step, as well as HEA2.907g, EHA7.370g, LA2.422g, add them to a 100mL round bottom flask, and add 50mL of ethyl acetate was reacted at 85°C for 24h. After the end, the product was added dropwise to a precipitant to precipitate, and a white solid was obtained, ...
Embodiment 2
[0024] Step 1: Step 1: Weigh 6.320g of methacryloyl chloride, dissolve it in 50mL of tetrahydrofuran (THF), and add it dropwise to 2-methylimidazole (4.105g) and three Ethylamine (6.07g) in 100mL THF solution, stirred, and the above process was carried out in a 250mL three-necked flask. After the dropwise addition, the stirring was continued at room temperature for 24-48 hours, and the reaction ended. The ammonium salt generated during the reaction is removed by suction filtration of the system, and the solvent is removed by rotary evaporation to obtain a free radical comonomer with an imidazole ring.
[0025] Step 2: Weigh 3.380g of the monomer containing imidazole ring and double bond synthesized in the first step, as well as 2.907g of HEA, 7.370g of EHA, and 2.422g of LA, and add them to a 100mL round bottom flask, and add 50mL of ethyl acetate was reacted at 85°C for 24h. After the end, the product was added dropwise to a precipitant to precipitate, and a white solid was...
Embodiment 3
[0028] The first step: Weigh 2-methylimidazole (4.105g) and triethylamine (6.07g), dissolve in 100mL tetrahydrofuran (THF), and add methacryloyl chloride ( 5.227g) in 50mL THF solution, stirred, and the above process was carried out in a 250mL three-necked flask. After the dropwise addition, the stirring was continued at room temperature for 24-48 hours, and the reaction ended. The ammonium salt generated during the reaction is removed by suction filtration of the system, and the solvent is removed by rotary evaporation to obtain a free radical comonomer with an imidazole ring.
[0029] Step 2: Weigh 3.380g of the monomer containing imidazole ring and double bond synthesized in the first step, as well as 2.907g of HEA, 7.370g of EHA, and 2.422g of LA, and add them to a 100mL round bottom flask, and add 50mL of ethyl acetate was reacted at 85°C for 24h. After the end, the product was added dropwise to a precipitant to precipitate, and a white solid was obtained, which was dri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com