Non-aqueous electrolytic solution, magnesium secondary battery of non-aqueous electrolytic solution
A non-aqueous electrolyte and magnesium secondary battery technology, applied in secondary batteries, circuits, electrical components, etc., to achieve the effect of easy preparation and high coulombic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Weigh 0.120g of tris(hexafluoroisopropoxy) borate into a vial with an electronic balance, take 2mL of ethylene glycol dimethyl ether into the above vial with a pipette gun, and then weigh 0.010g of anhydrous fluorine Magnesium chloride is placed in the above-mentioned vial containing the organic solvent and organoborane, and a magnet is added to carry out magnetic stirring for 10 hours. After being completely dissolved, it is prepared into a non-aqueous electrolyte.
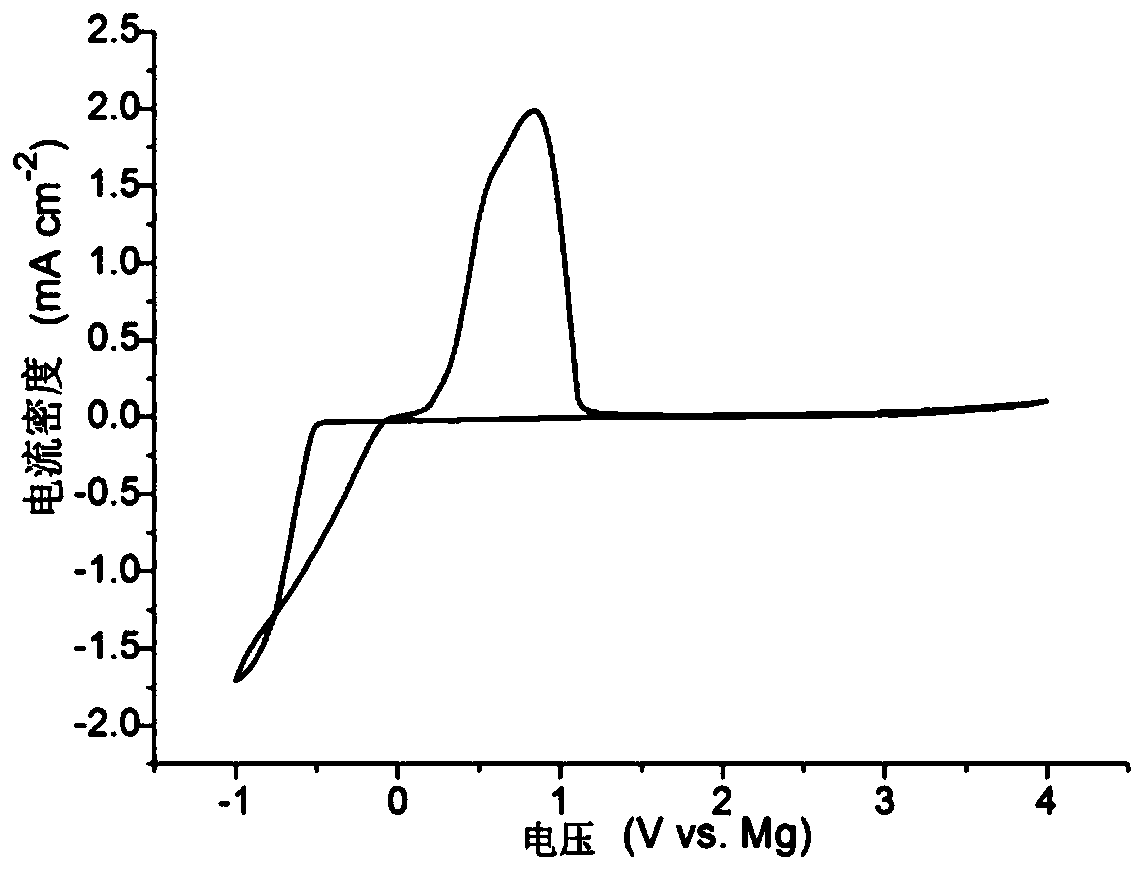
[0045] In a glove box full of argon, the electrolyte is used as the electrolyte of the assembled battery, the stainless steel sheet is the positive electrode, and the magnesium sheet is the negative electrode assembled into a standard button cell, and the battery is subjected to cyclic voltammetry (see figure 1 ), the scanning voltage range is -1-4.0V vs. Mg. The figure shows that the electrolyte has an excellent ability to reversibly deposit-dissolve magnesium, and that the stable voltage of the electroly...
Embodiment 2
[0047] Weigh 0.120g of tris(hexafluoroisopropoxy) borate into a vial with an electronic balance, take 2mL of ethylene glycol dimethyl ether into the above vial with a pipette gun, and then weigh 0.005g of anhydrous fluorine Magnesium chloride is placed in the above-mentioned vial containing the organic solvent and organoborane, and a magnet is added to carry out magnetic stirring for 10 hours. After being completely dissolved, it is prepared into a non-aqueous electrolyte.
[0048] In a glove box filled with argon, the electrolyte is used as the electrolyte of the assembled battery, the stainless steel sheet is used as the positive electrode, and the magnesium sheet is used as the negative electrode to assemble a standard button battery, and the battery is subjected to cyclic voltammetry test, and the scanning voltage range is - 1-4.0V vs. Mg. The electrolyte has an excellent ability of reversibly depositing and dissolving magnesium, and the stable voltage of the electrolyte i...
Embodiment 3
[0049] Weigh 0.120g of tris(hexafluoroisopropoxy) borate into a vial with an electronic balance, take 2mL of ethylene glycol dimethyl ether into the above vial with a pipette gun, and then weigh 0.020g of anhydrous fluorine Magnesium chloride is placed in the above-mentioned vial containing the organic solvent and organoborane, and a magnet is added to carry out magnetic stirring for 10 hours. After being completely dissolved, it is prepared into a non-aqueous electrolyte.
[0050] In a glove box filled with argon, the electrolyte is used as the electrolyte of the assembled battery, the stainless steel sheet is used as the positive electrode, and the magnesium sheet is used as the negative electrode to assemble a standard button battery, and the battery is subjected to cyclic voltammetry test, and the scanning voltage range is - 1-4.0V vs. Mg. The electrolyte has an excellent ability of reversibly depositing and dissolving magnesium, and the stable voltage of the electrolyte i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com