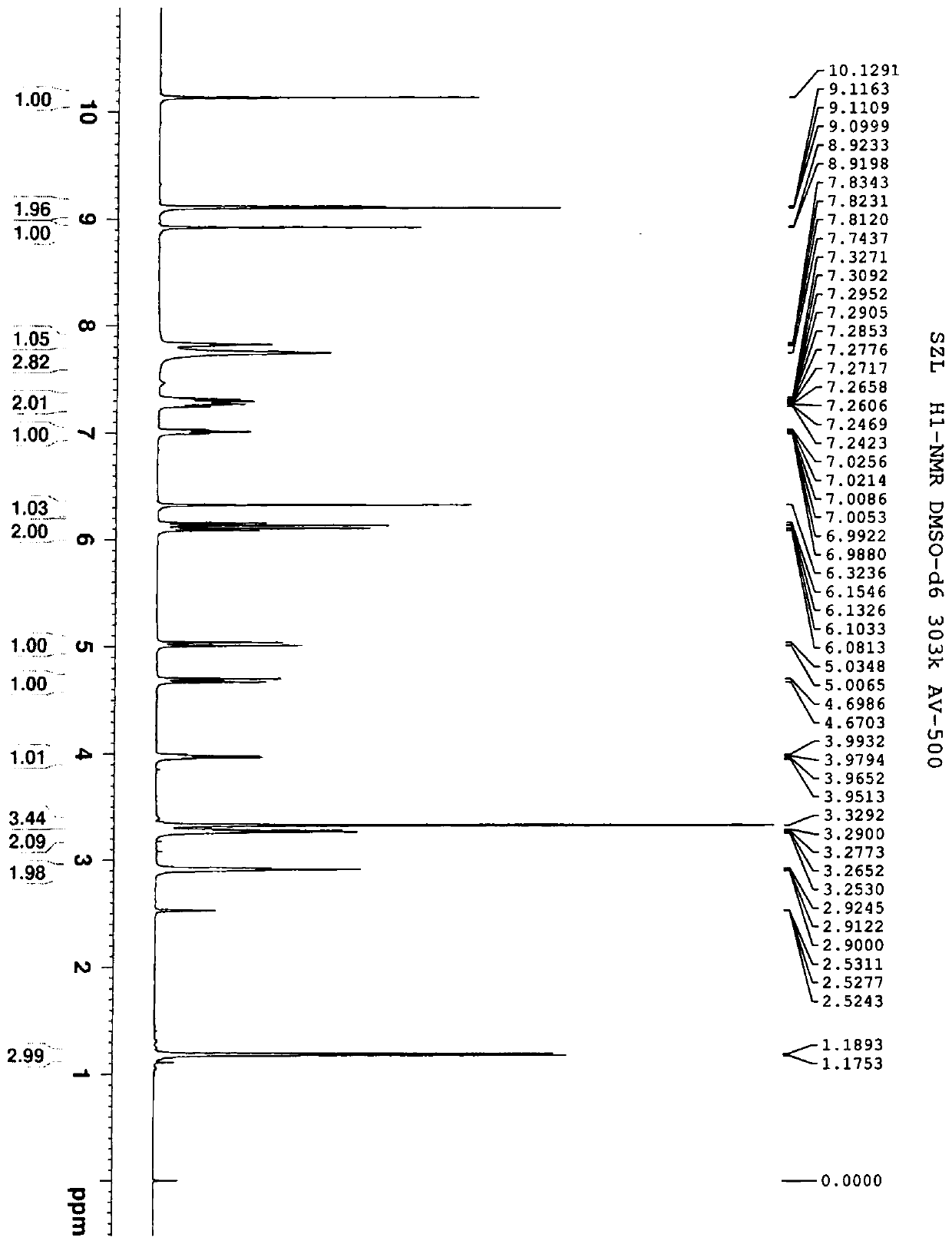
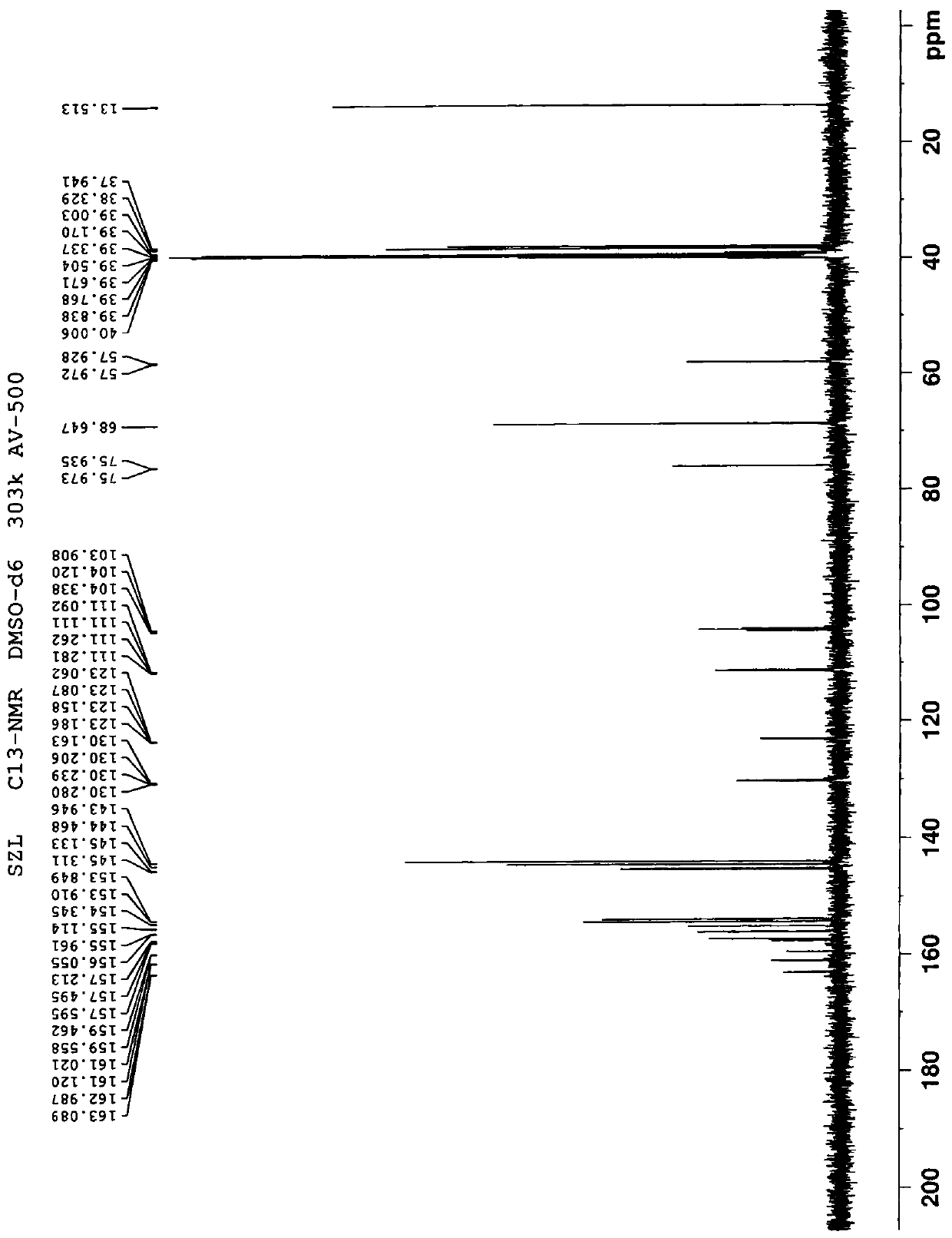
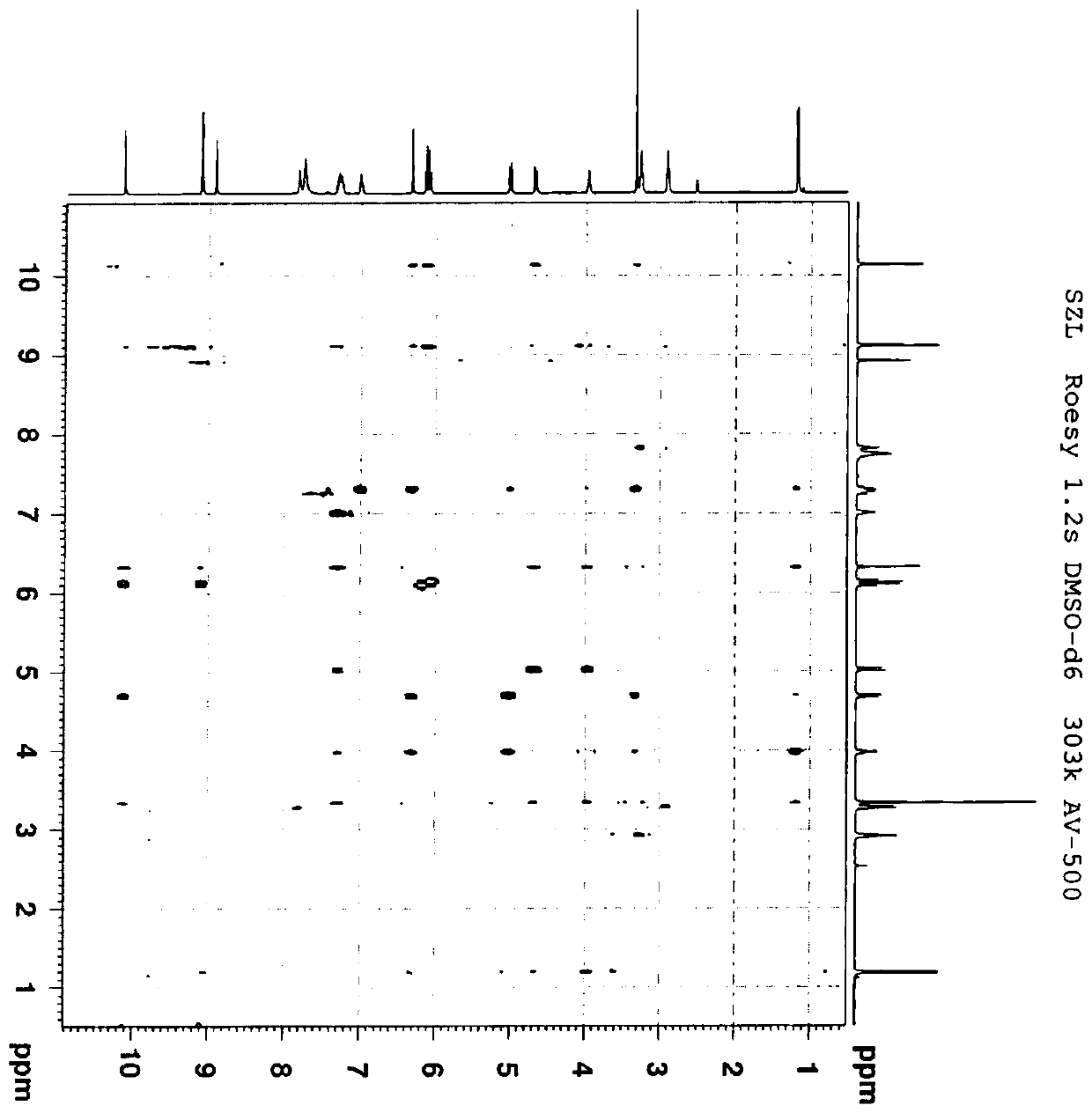
A water-soluble triazole compound
A triazole compound, water-soluble technology, applied in the field of water-soluble triazole compound and its preparation, can solve the problems of difficult synthesis of water-soluble compounds, poor drug stability, etc., achieve excellent water solubility, improve solubility and stability , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Synthesis of Compound 3
[0040] Chemical reaction formula:
[0041]
[0042] Steps:
[0043] In a 500 mL reaction flask, add 20 g of compound a and 200 mL of dichloromethane, stir, and then add 12.8 g of pyridine. Then 17.7 g of chloromethyl chloroformate was added dropwise at 10-20°C, after the dripping was completed, the temperature was raised to 20-25°C for 24 hours. Add 100 mL of water to the reaction solution, stir and separate the layers, and then wash the organic layer with 80 mL×2 with water. Collect the organic layer, dry over anhydrous sodium sulfate, and concentrate to dryness under reduced pressure to obtain 27.4 g of oil, which is compound 1. The yield was 87%.
[0044] In a 1L reaction flask, add 27.4g of compound 1, 23.4g of potassium iodide and 410mL of acetonitrile, stir, and then add 37.8g of compound X (voriconazole), and heat to reflux for 15h. Stop the reaction, cool to 20-25°C, filter to remove insoluble materials, and concentrate the filtrat...
Embodiment 2
[0050] Example 2 Synthesis of Compound 6
[0051] Chemical reaction formula:
[0052]
[0053] Steps:
[0054] In a 250 mL reaction flask, add 10 g of compound b and 100 mL of dichloromethane, stir, and then add 13.7 g of pyridine. Then 18.9 g of chloromethyl chloroformate was added dropwise at 10-20°C, and after the dripping was completed, the temperature was raised to 20-25°C for 24 hours. Add 50 mL of water to the reaction solution, stir and separate the layers, and then wash the organic layer with 30 mL×2 of water. Collect the organic layer, dry over anhydrous sodium sulfate, and concentrate to dryness under reduced pressure to obtain 18.9 g of oil, which is compound 4. The yield was 84%.
[0055] In a 500 mL reaction flask, add 18.9 compound 4, 24.3 g potassium iodide and 285 mL acetonitrile, stir, then add 39.3 g compound X (voriconazole), and heat to reflux for 15 hours. Stop the reaction, cool to 20-25°C, filter to remove insoluble materials, and concentrate the filtrate to...
Embodiment 3
[0059] Example 3 Synthesis of Compound 9
[0060] Chemical reaction formula:
[0061]
[0062] Steps:
[0063] In a 500 mL reaction flask, add 20 g of compound c and 200 mL of dichloromethane, stir, and then add 14.7 g of pyridine. Then 17.6g of chloromethyl chloroformate was added dropwise at 10-20°C, after the dripping was completed, the temperature was raised to 20-25°C for 24h reaction. Add 100 mL of water to the reaction solution, stir and separate the layers, and then wash the organic layer with 80 mL×2 with water. Collect the organic layer, dry over anhydrous sodium sulfate, and concentrate to dryness under reduced pressure to obtain 26.8 g of oil, which is compound 7. The yield was 85%.
[0064] In a 1 L reaction flask, add 26.8 g of compound 7, 22.8 g of potassium iodide and 400 mL of acetonitrile, stir, and then add 36.8 g of compound X (voriconazole), and heat to reflux for 15 hours. Stop the reaction, cool to 20-25°C, filter to remove insoluble materials, and concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com