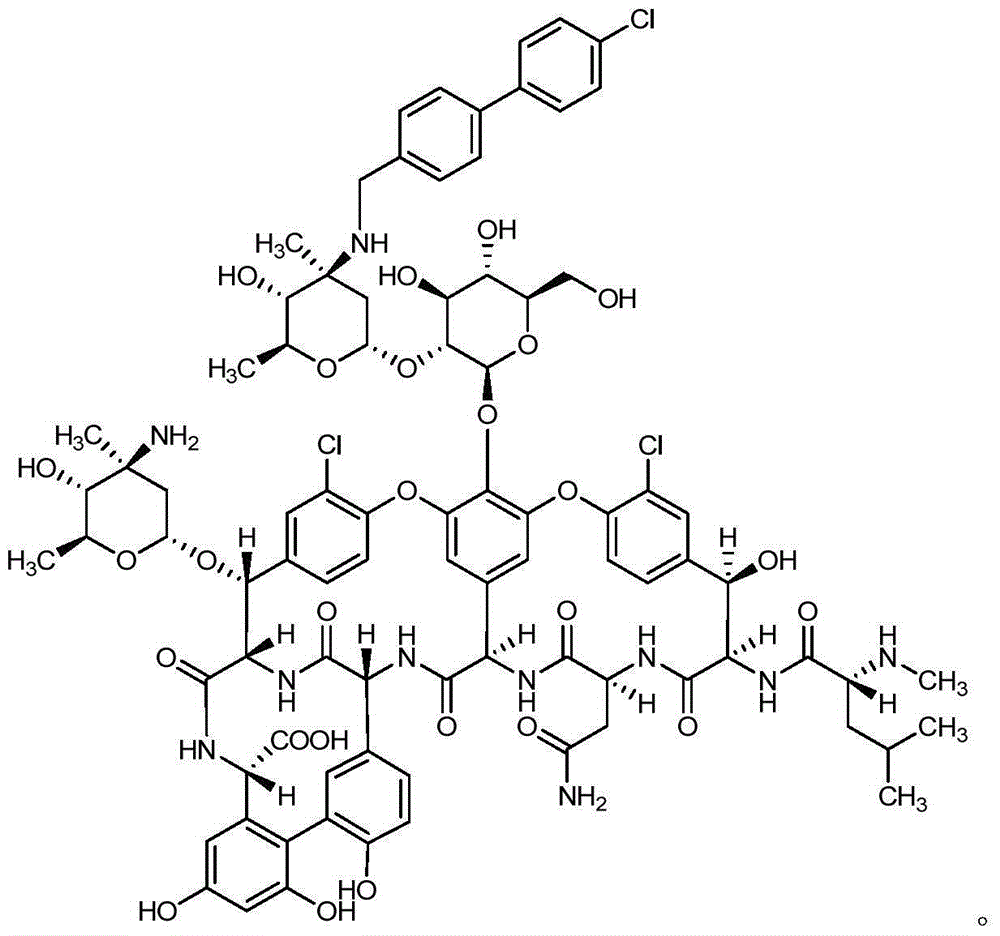
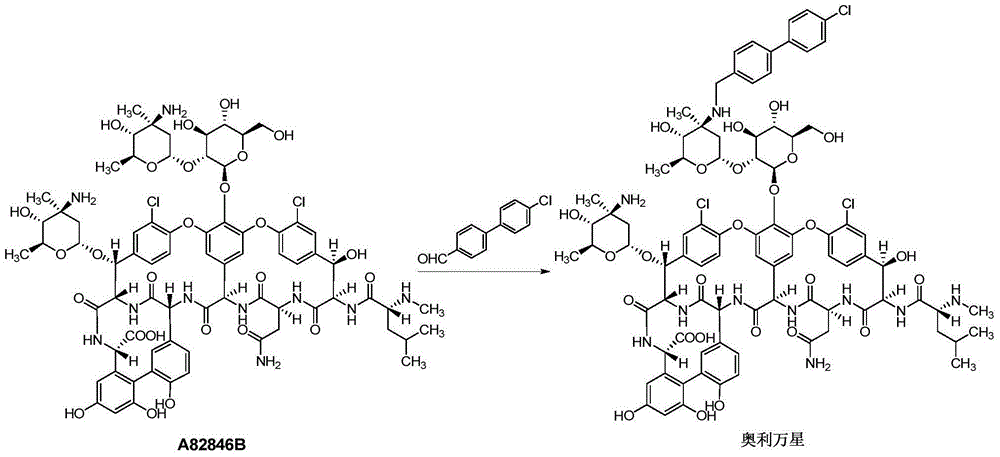
Reductive alkylation method for glycopeptide antibiotics
A technology of glycopeptide antibiotics and alkylation, which is applied in the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problems of low yield and many secondary substitution impurities, achieve high yield and control by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add A82846B (0.446g, 0.28mmol) to 45ml of anhydrous methanol, stir at room temperature for 0.5h, add DIEA (550ul, 3.3mmol, 11.9eq), stir at room temperature for 0.5h, add copper acetate monohydrate (56mg, 0.28mmol , 1eq) continued to stir for 2h, added 4'-chloro-4-biphenylcarbaldehyde (212.3mg, 0.98mmol, 3.5eq), and continued to stir for 24h. Under the protection of nitrogen, the system was cooled to 0-10°C, trifluoroacetic acid (278.9ul, 3.64mmol, 13eq) was added, and after the reaction was stirred for 1h under temperature control, TBAB (40mg, 0.46mmol, 1.6eq) was added to the reaction in 4 times solution (0.4eq / time / hour), add and react for 1h. The reaction solution was taken for HPLC detection. After the reaction is finished, add 1mol / L sodium hydroxide solution dropwise to adjust the pH of the system to 9.0, concentrate under reduced pressure to leave about 15ml, add 15ml of acetonitrile dropwise into the system, stir and crystallize for 1 hour, filter with suction...
Embodiment 2
[0031] Add A82846B (0.43g, 0.27mmol) to 43ml of anhydrous methanol, stir at room temperature for 0.5h, add DIEA (267ul, 1.62mmol, 6eq), stir at room temperature for 0.5h, add copper acetate monohydrate (53.9mg, 0.27mmol , 1eq) continued to stir for 2h, added 4'-chloro-4-biphenylcarbaldehyde (204.75mg, 0.945mmol, 3.5eq), and continued to stir for 24h. Under the protection of nitrogen, the system was cooled to 0-10°C, trifluoroacetic acid (206.9ul, 2.7mmol, 10eq) was added, and after stirring for 1 hour under temperature control, TBAB (37.57mg, 0.432mmol, 1.6eq) was added to the In the reaction solution (0.4eq / time / hour), the reaction was completed for 1h. The reaction solution was taken for HPLC detection. After the reaction is finished, add 1mol / L sodium hydroxide solution dropwise to adjust the pH of the system to 9.0, concentrate under reduced pressure to leave about 15ml, add 15ml of acetonitrile dropwise into the system, stir and crystallize for 1 hour, filter with suctio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com