Non-aqueous electrolyte solution and electricity storage device in which same is used
一种非水电解液、蓄电设备的技术,应用在非水电解质蓄电池、非水电解质电池、非水电解质等方向,能够解决电池性能下降等问题,达到提高放电容量维持率、改善低温输入特性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
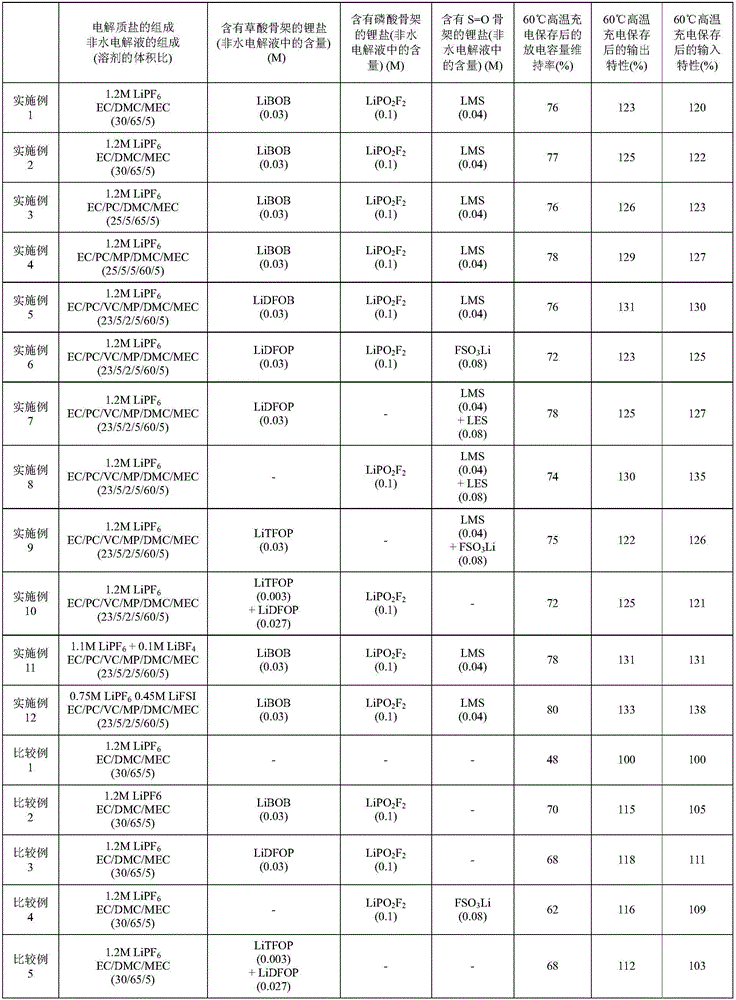
Embodiment 1~12、 comparative example 1~5
[0152] 〔Manufacture of lithium-ion secondary battery〕
[0153] 94 mass% LiNi 1 / 3 mn 1 / 3 co 1 / 3 o 2 , 3% by mass of acetylene black (conductive agent) were mixed, added to the solution obtained by dissolving 3% by mass of polyvinylidene fluoride (binder) in 1-methyl-2-pyrrolidone in advance and mixed to prepare Positive electrode mixture paste. This positive electrode mixture paste was applied to one side of an aluminum foil (current collector), dried and pressurized, and then cut into a predetermined size to produce a strip-shaped positive electrode sheet. The density of the part of the positive electrode other than the current collector is 3.6 g / cm 3 .
[0154] In addition, 10% by mass of silicon (single substance), 80% by mass of artificial graphite (d 002 =0.335nm, negative electrode active material), 5% by mass of acetylene black (conductive agent) are mixed, added to make 5% by mass of polyvinylidene fluoride (binder) dissolved in 1-methyl-2-pyrrolidone in advance ...
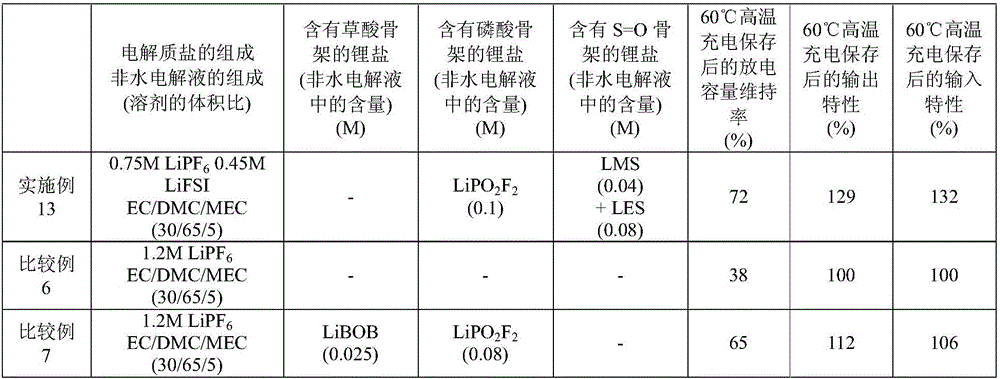
Embodiment 13 and comparative example 6、7
[0184] Lithium manganate nickel salt (LiNi 1 / 2 mn 3 / 2 o 4 ) (positive electrode active material) instead of the positive electrode active material used in Example 1 and Comparative Examples 1 and 2, to produce a positive electrode sheet.
[0185] Mix 94% by mass of lithium nickel manganate and 3% by mass of acetylene black (conductive agent), and add 3% by mass of polyvinylidene fluoride (binder) into 1-methyl-2-pyrrolidone in advance The resulting solution was mixed to prepare a positive electrode mixture paste.
[0186] This positive electrode mixture paste was applied to one side of the aluminum foil (current collector), dried and pressurized, and then cut into a predetermined size to make a positive electrode sheet. The end-of-charge voltage at the time of battery evaluation was set to 4.9 V, except that the end-of-discharge voltage was set to 2.7 V, laminated batteries were produced in the same manner as in Example 1 and Comparative Example 1, and battery evaluation wa...
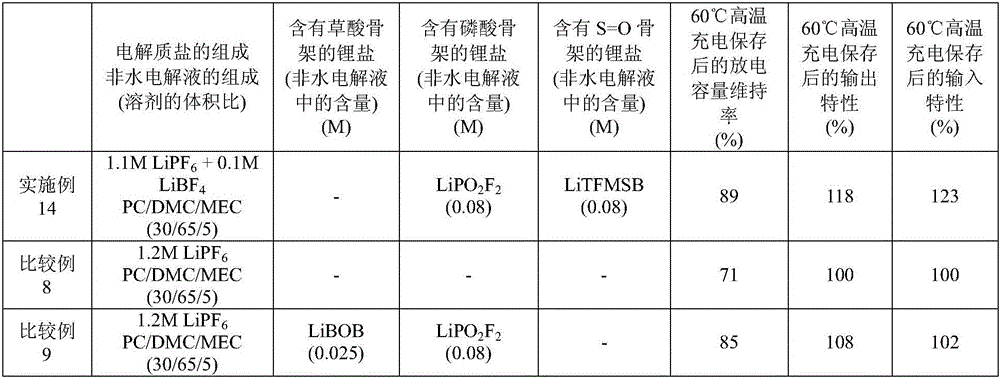
Embodiment 14 and comparative example 8、9
[0190] Lithium titanate (Li 4 Ti 5 o 12 ) (negative electrode active material) instead of the negative electrode active material used in Example 1 and Comparative Examples 1 and 2, to produce a negative electrode sheet.
[0191]Mix 80% by mass of lithium titanate and 15% by mass of acetylene black (conductive agent), and add to 1-methyl-2-pyrrolidone by dissolving 5% by mass of polyvinylidene fluoride (binder) in advance. solution and mixed to prepare negative electrode mixture paste.
[0192] This negative electrode mixture paste is applied to one side of the copper foil (current collector), dried and pressurized, then cut into a predetermined size to make a negative electrode sheet, and the final charge voltage during battery evaluation is set to 2.8V, except that the end-of-discharge voltage was set at 1.2V, and the composition of the nonaqueous electrolyte was changed to a predetermined composition, laminated batteries were produced in the same manner as in Example 1 an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| lattice spacing | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com