Synthesis method of organic phosphorus compound
A synthesis method and selected technology, applied in the field of synthesis of organophosphorus compounds, can solve the problems of unsatisfactory industrial application, long reaction route, and high requirements for production equipment, and achieve unique catalytic activity, fewer steps, and easy separation and purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
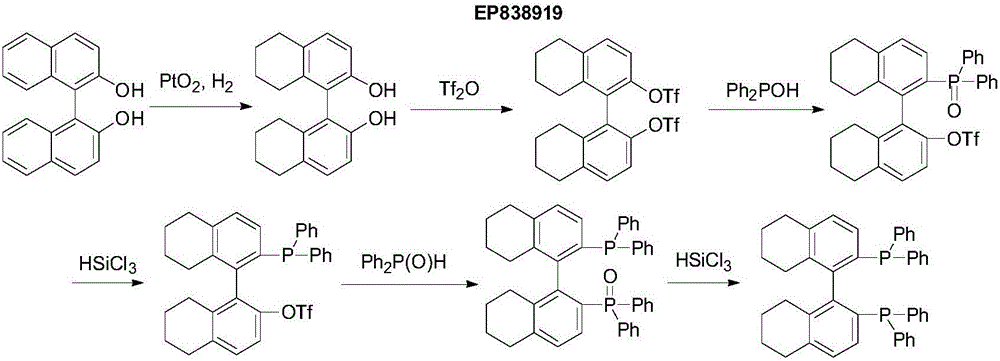
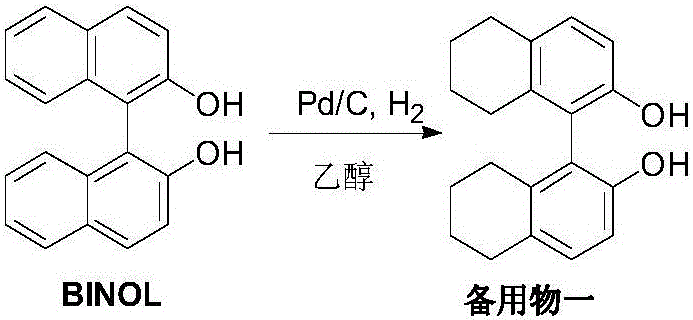
[0031] Add 50g(R)-BINOL, 15g 5%Pd / C (50%wet), 300mL ethanol to a 1L autoclave, seal the autoclave, vacuumize, replace with nitrogen once, then replace with hydrogen three times, adjust the pressure to 60atm, Raise the temperature to 75-80°C, keep the temperature for 8 hours, cool the autoclave to room temperature, filter, and concentrate the filtrate to dryness for later use to obtain a viscous standby product 1.
Embodiment 2
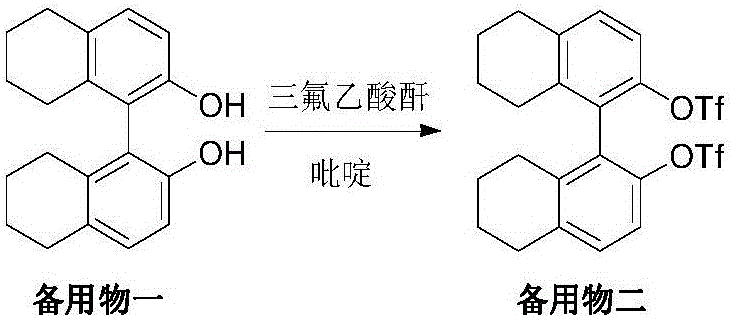
[0033] Dissolve the spare material in Example 1 in 300mL of dichloromethane, add it into 32mL of pyridine solution, cool to below 5°C, add 108g of trifluoroacetic anhydride dropwise, the temperature does not exceed 25°C during the dropwise addition, and stir at room temperature for 24 hours after the dropwise addition , filtered off the insoluble matter, concentrated the filtrate, and when a small amount of solvent remained, add methanol to crystallize, filter, and dry in vacuum at 60-70°C to obtain 70g of yellow standby product 2 with a yield of 72% and a purity of 99.2%.
Embodiment 3
[0035] With the 1.1g spare material two in the example 2, 0.0448gPd (OAc) (10mol%), 0.085gDPPE (10mol%), join in the 5mLDMF solvent, vacuumize, argon replacement is entered reactor, then 0.57gDIPEA and 0.82 Add 1 g of diphenylphosphine to the reaction system, raise the temperature to 120°C with stirring, keep warm and reflux for 72 hours, cool to room temperature, add the reaction solution into 30mL methanol, filter, rinse the filter cake with a small amount of methanol, Dry under vacuum to obtain 0.58g off-white solid, yield 46%, melting point 207~208 ℃, 31 P-NMR (CDCl 3 ):-16.31(S), [] D +69.7 (C 0.5 Toluene).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com