A kind of synthetic method of organophosphorus compound
A synthesis method and selected technology, applied in the field of synthesis of organophosphorus compounds, can solve problems such as high requirements for production equipment, inability to meet industrial applications, and long reaction routes, and achieve the effects of easy separation and purification, unique catalytic activity, and fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
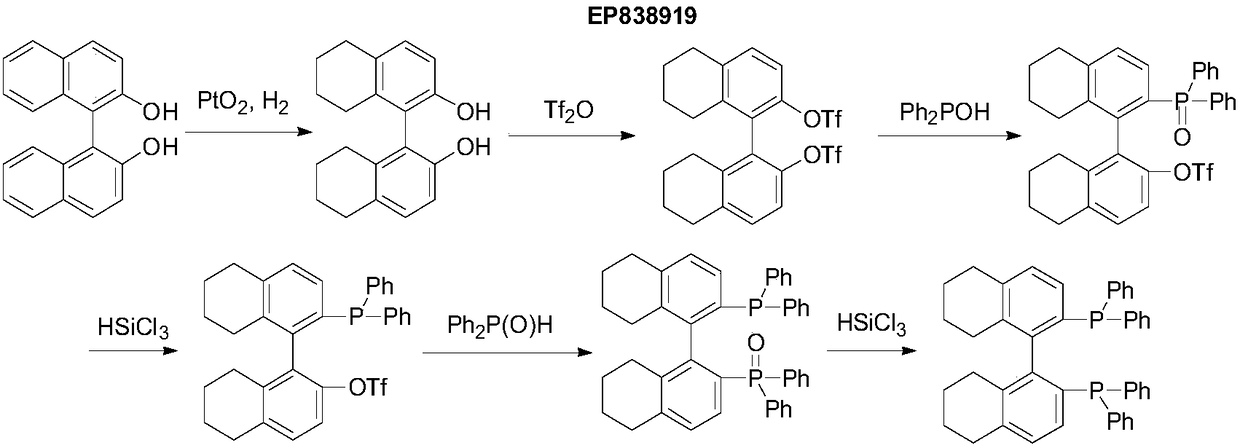
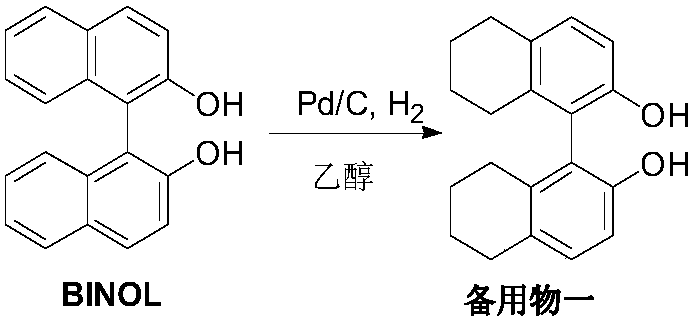
Embodiment 1
[0031] Add 50g (R)-BINOL, 15g 5%Pd / C (50%wet), 300mL ethanol to a 1L autoclave, seal the autoclave, evacuate, replace with nitrogen once, and replace with hydrogen 3 times, adjust the pressure to 60atm, The temperature was raised to 75-80° C., the reaction was kept for 8 hours, the autoclave was cooled to room temperature, filtered, and the filtrate was concentrated to dryness for later use, to obtain a viscous standby material 1.
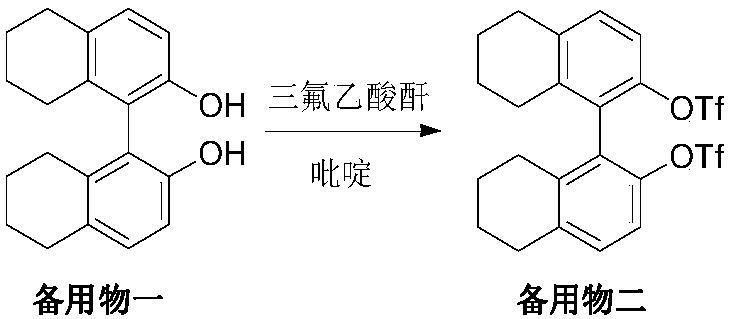
Embodiment 2
[0033] Dissolve the standby material in Example 1 in 300 mL of dichloromethane, add 32 mL of pyridine solution, cool to below 5 ° C, add 108 g of trifluoroacetic anhydride dropwise, the temperature does not exceed 25 ° C during the dropwise addition, and stir at room temperature for 24 hours after the dropwise addition. , filtered off the insoluble matter, concentrated the filtrate, and when a small amount of solvent remained, added methanol for crystallization, filtered, and vacuum-dried at 60-70° C. to obtain 70 g of yellow ready-to-use product 2 with a yield of 72% and a purity of 99.2%.
Embodiment 3
[0035] In Example 2, 1.1g of standby material two, 0.0448g of Pd(OAc) (10mol%), 0.085g of DPPE (10mol%) were added to 5mL of DMF solvent, evacuated, and argon was replaced into the reactor, then 0.57g of DIPEA and 0.82 g diphenylphosphine was added to the reaction system, heated to 120°C with stirring, kept at reflux for 72 hours, cooled to room temperature, the reaction solution was added to 30 mL of methanol, filtered, and the filter cake was rinsed with a small amount of methanol at 60-70°C Under vacuum drying, 0.58g of off-white solid was obtained, the yield was 46%, and the melting point was 207-208°C. 31 P-NMR (CDCl 3 ):-16.31(S), [] D +69.7 (C 0.5 Toluene).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com