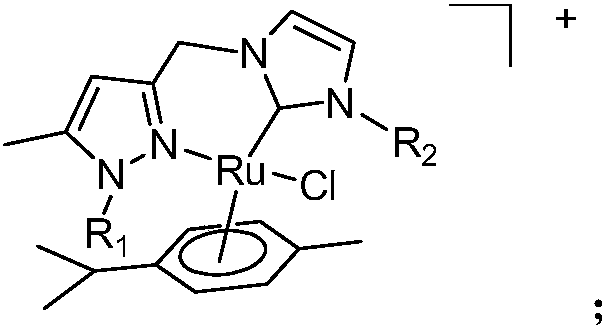
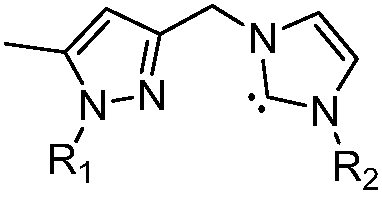
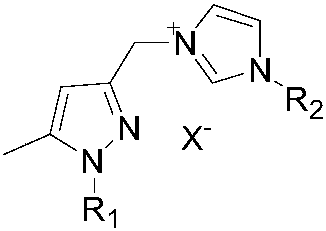
A pyrazole-functionalized nitrogen-heterocyclic carbene ruthenium compound with anticancer activity and its preparation method and application
A nitrogen-heterocyclic carbene and ruthenium compound technology, applied in ruthenium organic compounds, platinum group organic compounds, organic active ingredients, etc., can solve the problems of drug resistance and dose-limiting toxicity of cancer cells, and achieve inhibition of division and reproduction, Environmentally friendly and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Ethyl 5-methyl-1-hydro-pyrazolecarboxylate (30.8g, 200mmol) was dissolved in 200mL of dry tetrahydrofuran solution, and deoiled sodium hydride (4.8g, 200mmol) was added slowly under ice bath conditions, and sodium hydride was added completely Afterwards, the temperature was raised to 50° C., stirred for 1 hour, and then cooled to room temperature. Then iodomethane (28.2 g, 200 mmol) was dissolved in 100 mL of tetrahydrofuran, and slowly added dropwise to the reaction. After the dropwise addition was completed, the mixture was heated to 50° C. and continued to stir for 2 hours. After the reaction was complete, cool to room temperature, remove THF under reduced pressure, then add 100 mL of water, extract with ethyl acetate (100 mL×3), combine the organic layers, dry, and remove ethyl acetate under reduced pressure to obtain 5-methyl-1-methanol Ethyl-pyrazolecarboxylate 26.6g, 85%. Add lithium aluminum hydride (3.8g, 100mmol) and 200mL dry tetrahydrofuran into...
Embodiment 2
[0032]
[0033] Ligand HL2PF 6 Synthesized with reference to the method of Example 1, ethyl bromide replaces methyl iodide. At a temperature of 50°C, the ligand HL2PF was added 6 , 454mg (1mmol), acetonitrile 10mL, silver oxide 116mg (0.5mmol), react for 4 hours, then add [Ru(p-cymene)Cl 2 ] 2 306mg (0.5mmol) continued to react at room temperature for 2 hours, centrifuged, concentrated the filtrate to 2mL, added diethyl ether to precipitate a yellow solid, washed the yellow solid twice with diethyl ether, then dissolved it with acetonitrile, slowly added diethyl ether, and recrystallized to obtain 333 mg of nitrogen heterocycle Carbene ligand-coordinated ruthenium compound 2, yield 46%. 1 H NMR (400MHz, CD 3CN):7.54(s,1H),7.16(s,1H),7.14(s,1H),6.99(s,1H),6.36(s,1H),5.86,5.78,5.43,5.34(both d,4H ),5.31,4.96(both d,2H),4.86-4.69(m,2H),2.48(s,3H),2.36(s,3H),2.25(s,3H),2.02(s,3H),1.98 -1.96 (m, 1H), 1.60 (s, 3H), 1.40 (t, 3H), 1.14, 0.58 (both d, 6H) ppm. 13 C NMR (CD ...
Embodiment 3
[0035]
[0036] Ligand HL3PF 6 Synthesize with reference to the method of Example 1, isopropyl bromide replaces methyl iodide. At a temperature of 50°C, the ligand HL3PF was added 6 , 468mg (1mmol), acetonitrile 10mL, silver oxide 116mg (0.5mmol), react for 4 hours, then add [Ru(p-cymene)Cl 2 ] 2 306 mg (0.5 mmol) continued to react at room temperature for 2 hours, centrifuged, concentrated the filtrate to 2 mL, added diethyl ether to precipitate a yellow solid, washed the yellow solid twice with diethyl ether, then dissolved it with acetonitrile, slowly added diethyl ether, and recrystallized to obtain 353 mg of nitrogen heterocycle Carbene ligand-coordinated ruthenium compound 3, yield 53%. 1 H NMR (CD 3 CN):7.50(s,1H),7.22(s,1H),7.18(s,1H),6.80(s,1H),6.30(s,1H),5.82,5.72,5.41,5.30(both d,4H ), 5.26, 4.86 (both d, CH 2 ,2H),4.04(m,1H),2.42(s,CH 3 ,3H),2.34(s,3H),2.26(s,3H),2.12(s,3H),1.98-1.92(m,1H),1.62(s,3H),1.56(d,6H),1.16, 0.60 (both d, 6H) ppm. 13 C NMR (CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com