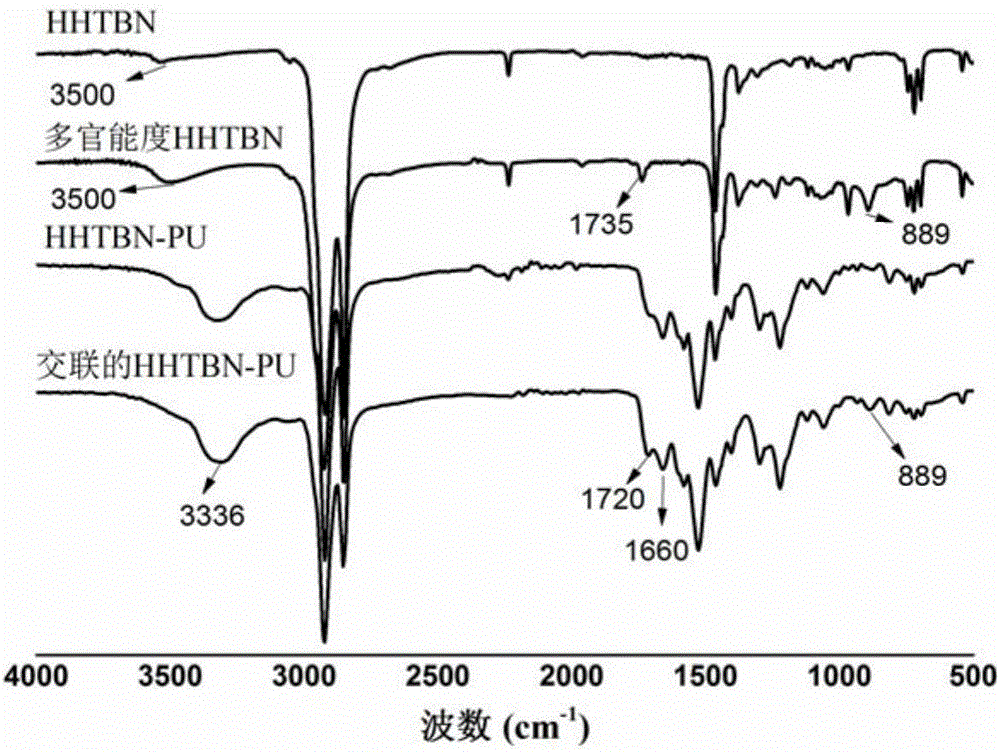
Cross-linked hydrogenated butyronitrile type polyurethane
A hydrogenated nitrile and polyurethane technology, which is applied in the field of cross-linked hydrogenated nitrile polyurethane and its preparation, can solve the problems of low mechanical properties, poor ozone aging resistance, and affecting product performance, and overcome low mechanical strength , broaden the scope of application, overcome the effects of poor aging resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
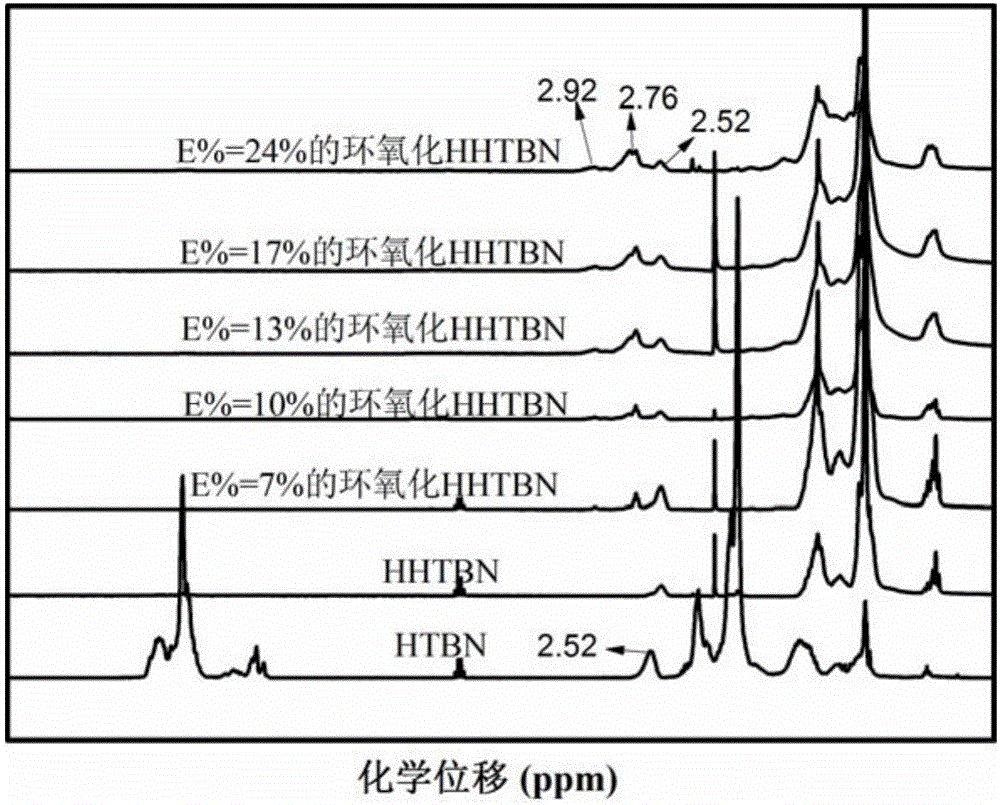
[0035] (1) Get hydroxyl-terminated liquid nitrile rubber (HTBN) and be dissolved in xylene, be mixed with the solution of mass fraction 10wt%; Get 300g above-mentioned solution in the 500ml there-necked bottle, add 29.88g acetic acid, then add dropwise hydrogen peroxide solution ( Aladdin reagent, mass fraction 30%) 84.66g, stirred and reacted at 65°C for 4h. The reacted mixed solution is precipitated, washed, and dried to obtain epoxidized hydroxyl-terminated liquid nitrile rubber (EHTBN), and the epoxy degree E%=7% is calculated by nuclear magnetic detection.
[0036] (2) use xylene as solvent, EHTBN is made into the glue solution that mass concentration is 10%, adds glue solution 250g in autoclave, adds Wilkinson catalyst 0.2g again, ligand triphenylphosphine 0.6g, The temperature was 110° C. and the hydrogen pressure was 4 MPa, and the hydrogenation reaction was carried out for 8 hours. After the reaction, a hydroxyl-terminated liquid hydrogenated nitrile rubber (E-HHTBN) ...
Embodiment 2
[0040]The step (1) epoxidation time is extended to 6h, and other conditions remain unchanged, namely obtain EHTBN of E%=10%; The higher the epoxidation degree of EHTBN will make the product hydroxyl obtained by step (3) ring-opening reaction Value is higher; Therefore, the isocyanate content of step (4) gained prepolymer reduces, and its value is 5.49% after measuring, calculates according to material proportion, needs to add MOCA quality and be 2.62g.
[0041] Other operations are the same as in Example 1.
Embodiment 3
[0043] Prolong step (1) epoxidation time to 8h, other conditions remain unchanged, namely obtain the EHTBN of E%=13%; The epoxidation degree of EHTBN is higher, will make the product hydroxyl that step (3) ring-opening reaction obtains Value is higher; Therefore, the isocyanate content of step (4) gained prepolymer reduces, and its value is 4.89% after measuring, calculates according to material proportion, needs to add MOCA quality and is 2.33g.
[0044] Other operations are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com