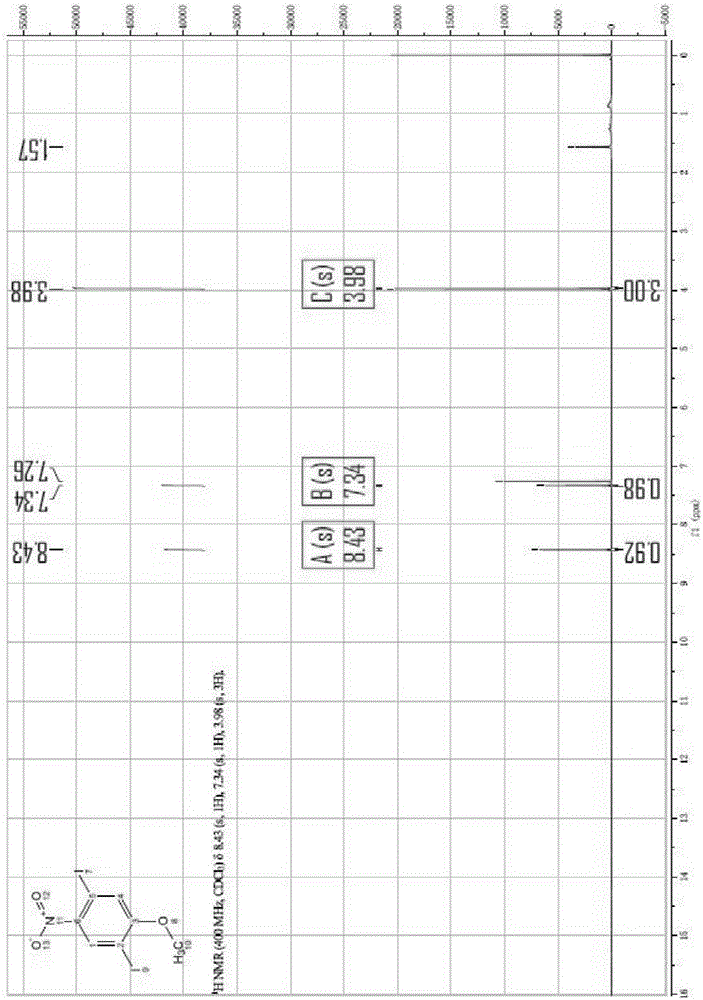
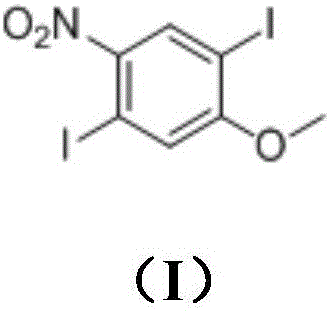
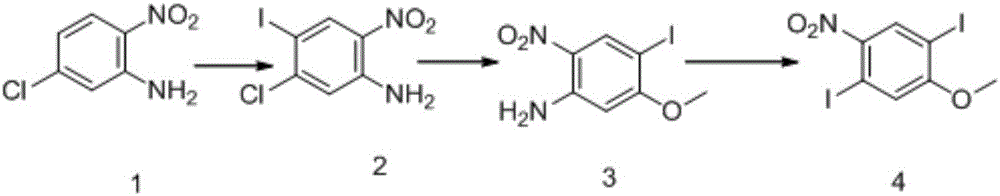
Method for synthesizing 1,4-diiodo-2-methoxy-5-nitrobenzene
A synthetic method, methoxy technology, applied in the preparation of amino compounds, chemical instruments and methods, and the preparation of amino compounds from amines, etc., can solve the problems of non-environmental protection, high cost, and many by-products, and achieve easy scale-up production and selection The effect of high stability and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The technical solutions of the present invention are described below through specific examples, but the protection scope of the present invention is not limited thereto. The raw materials used and the actual are commercially available products.
[0026] (1) Preparation of Compound 2
[0027]
[0028] Add compound 1, 2-nitro-5-chloro-aniline (1500g, 8.7mol) to 15L of acetic acid, then add N-iodosuccinimide NIS (1957.0g, 8.7mol), and heat to 55°C After 2 hours of reaction, TLC detected that the reaction was complete. The reaction solution was cooled to 10°C, filtered, and the filter cake was washed with acetic acid, water and saturated sodium bicarbonate solution, and then washed with water until neutral. The crude product was dried to obtain Compound 2, namely 2-nitro-4-iodo-5-chloroaniline (2200 g, 7.4 mol), with a yield of 85%.
[0029] (2) Preparation of compound 3
[0030]
[0031] Compound 2, 2-nitro-4-iodo-5-chloroaniline (2200g, 7.4mol) was added to 7.3L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com