Fusion protein containing collagen binding structure domain
A technology combining structural domains and fusion proteins, applied in the biological field, can solve the problems of multifunctional fusion protein design, preparation and application difficulties, and achieve the effects of avoiding immune response, reducing toxic side effects, and reducing pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
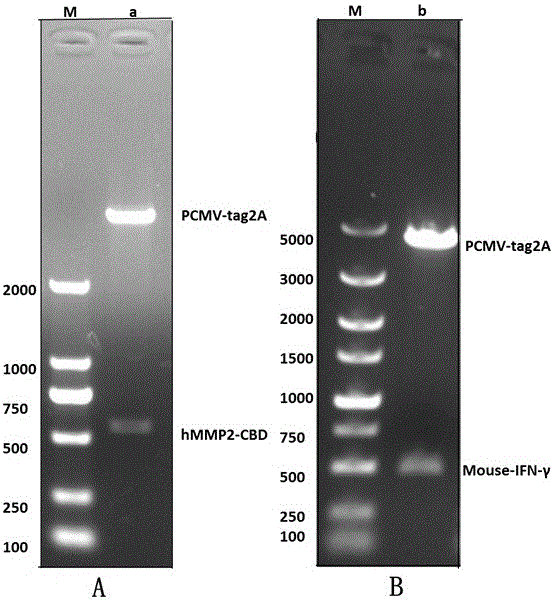
[0063] Example 1: Cloning of human CBD gene and cloning of mouse IFN-γ gene
[0064] The gene sequence of CBD was cloned from the existing human MMP2.
[0065] Cloning of the IFN-γ gene:
[0066] (1). Use LA enzyme to Xhol with EcoR1 is the enzyme cutting site, and the primers were synthesized by Wuhan Qingke Biotechnology Co., Ltd., and diluted with pure water to a working solution concentration of 10 μM. The IFN-γ gene sequence on which the primers were designed was derived from NCBI Reference Sequence: NM_008337.3).
[0067] (2). Preparation of template: RNA was extracted from mouse cells, and then reverse-transcribed to obtain cDNA as a template for PCR reaction
[0068] (3).PCR reaction system: 50ul reaction system contains: ddH 2 O 37.5ul; 10×LA buffer 5ul; 10mMdNTP 4ul; Primer (F+R) 2ul; mcDNA 1ul; LA enzyme 0.5ul. PCR reaction parameters: 95°C for 1 min; 94°C for 30 s, 57.9°C for 30 s, 72°C for 30 s, 40 cycles. 72°C for 5 min; 16°C for ∞.
[0069] (4). Af...
Embodiment 2
[0076] Example 2: Insert the cloned human MMP2 CBD and CDS region sigpeptide of the mouse IFN-γ DNA sequence into the expression vector PET28a(+) between EcoR1 and HindIII restriction sites. Using the method of fusion and recombination.
[0077] (1). First design the primers for fusion recombination.
[0078] (2). Fragment PCR, fragment PCR reaction system: 50ul reaction system, ddH 2 O 37.5ul; 10×LA buffer 5ul; 10mMdNTP 4ul; Primer (F+R) 2ul; plasmid 1ul; LA enzyme 0.5ul. PCR reaction parameters: 95°C for 5min; 94°C for 30s, 55°C for 30s, 72°C for 30s, 40 cycles. 72°C for 5 min; 16°C∞. After PCR cloning PCR is completed, take 2ul PCR product + 2ul 10×loading buffer, and carry out 1% agarose gel electrophoresis detection.
[0079] (3). PCR reaction system for vector linearization: 50ul reaction system contains: ddH 2 O 33ul; 10×KODbuff 5ul; 2mMdNTP 5ul; 25Mm MgSO4: 3ul; Primer (F+R) 2ul; plasmid 1ul; KOD enzyme 1ul. PCR reaction parameters: 95°C for 1 min; 95°C...
Embodiment 3
[0087] Implementation Example 3: Insert the sig peptide of the CDS region of the IFN-γ DNA sequence of the cloned mouse into the upstream of the CDS region of the DNA sequence of the CBD of human MMP2 in PET-28(a)+CBD
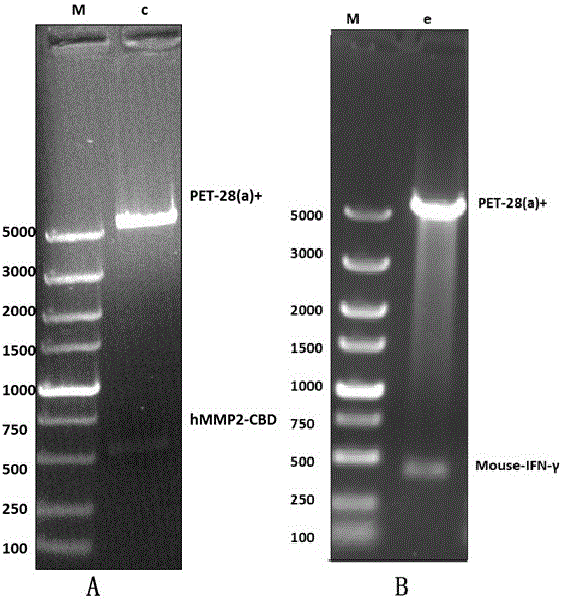
[0088] The operation method is still fusion and recombination, PET-28(a)+CBD is used as a carrier, PET-28(a)+IFN-γ is used as a fragment, the operation is the same as PET-28(a)+CBD, PET-28(a)+IFN - build of gamma.
[0089] image 3 After cloning the PET-28(a)+ IFN-γ-CBD plasmid, run a 1% DNA gel identification map, where the M band represents the DL5000 marker, and the e band represents the constructed PET- After the 28(a)+ IFN-γ-CBD plasmid was digested by ECORⅠ and HindⅢ, PET-28(a)+ and IFN-γ-CBD were excised respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com