A kind of organic photochromic material based on diarylethene and its preparation method and application
A photochromic material and diarylethene technology, which is applied in the field of materials science, can solve the problems of lack of spectral performance of ion-gated molecular switches, complex synthesis route and high cost, and achieve fast photoresponse ability, simple and excellent synthesis method. The effect of fatigue resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 (the synthesis of organic photochromic material C3-DAE):
[0029] Synthesis of compound C3-DAE
[0030]
[0031] The preparation method of 1-(5-chloro-2-methyl-3-thienyl)-2-(5-formyl-2-methyl-3-thienyl)cyclopentene (compound 1) refers to literature (H .Tian, et al., Tetrahedron, 2011,67,915-921), the preparation method of triaminoguanidine chloride salt (compound 2) is referred to literature (H.Krommer, et al., Chem.Abstr., 1986,104 , 206730).
[0032] In the dark, at room temperature, sequentially add 1-(5-chloro-2-methyl-3-thienyl)-2-(5-formyl-2-methyl-3-thienyl) into a 50mL single-necked bottle ) cyclopentene (0.882g, 2.73mmol), triaminoguanidine chloride salt (0.128g, 0.912mmol), 15mL ethanol and 4mL water, slowly warming up, stirring under reflux for 4h, naturally cooling to room temperature, filtering, The filter cake was washed with ether and dried to obtain 0.630 g of golden yellow powdery solid C3-DAE with a yield of 65.5%. 1 HNMR (400MHz,...
Embodiment 2
[0033] Embodiment 2 (photochromic performance of organic photochromic material C3-DAE):
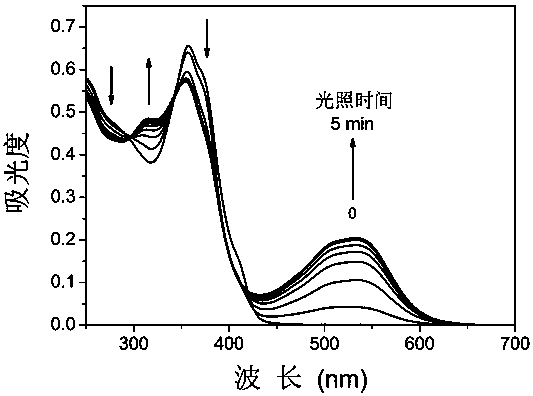
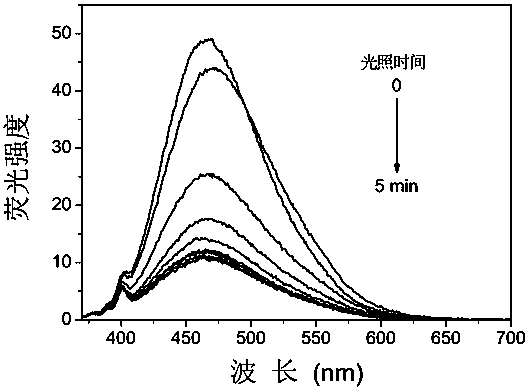
[0034] The compound C3-DAE obtained above was dissolved in acetonitrile, and the concentration was 10 μmol L -1 acetonitrile solution. Add 2.5mL of the solution to be tested into a 1cm×1cm×4cm quartz cuvette with stirring, and use a 365nm monochromatic light source to treat it according to different time lengths (10s, 30s, 60s, 90s, 120s, 180s, 240s, 300s) The solution was irradiated, and the absorption spectrum was measured with a UV-Vis spectrophotometer, the results were as follows figure 1 shown. Under the irradiation of 365nm ultraviolet light, a new absorption peak appeared in the absorption spectrum at 530nm, and gradually increased to reach the photostable state with the prolongation of time, and the color of the solution also changed from colorless to pink. Under the irradiation of visible light (≥550nm), the absorption spectrum returned to the original state and the color of ...
Embodiment 3
[0035] Embodiment 3 (copper ion controls the spectral performance of organic photochromic material C3-DAE):
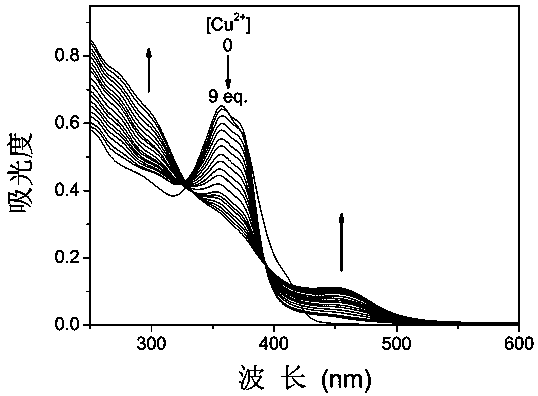
[0036] The compound C3-DAE obtained above was dissolved in acetonitrile, and the concentration was 10 μmol L -1 acetonitrile solution. Add 2.5mL of the solution to be tested into a 1cm×1cm×4cm quartz cuvette with stirring, and then add different concentrations of Cu with a micro-syringe 2+ (Cupric chloride) After mixing evenly for 2 minutes, test its absorption spectrum, the result is as follows image 3 shown. With Cu 2 + With continuous addition, the colorless solution gradually turned yellow. Using 365nm monochromatic light source to irradiate the complex solution for a long time will not cause changes in the absorption spectrum, and the color of the solution will not change either. It shows that the spectral properties of compound C3-DAE can be controlled by Cu 2+ Regulated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com