Cilostazol sustained-release capsule compound and preparation method thereof
A technology of cilostazol and sustained-release capsules, which is applied in the field of cilostazol sustained-release capsule compositions and its preparation, can solve problems such as difficulty in large-scale production, side effects of patients, and inconvenient dosage, and achieve product controllability Strong, easy to industrialize, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
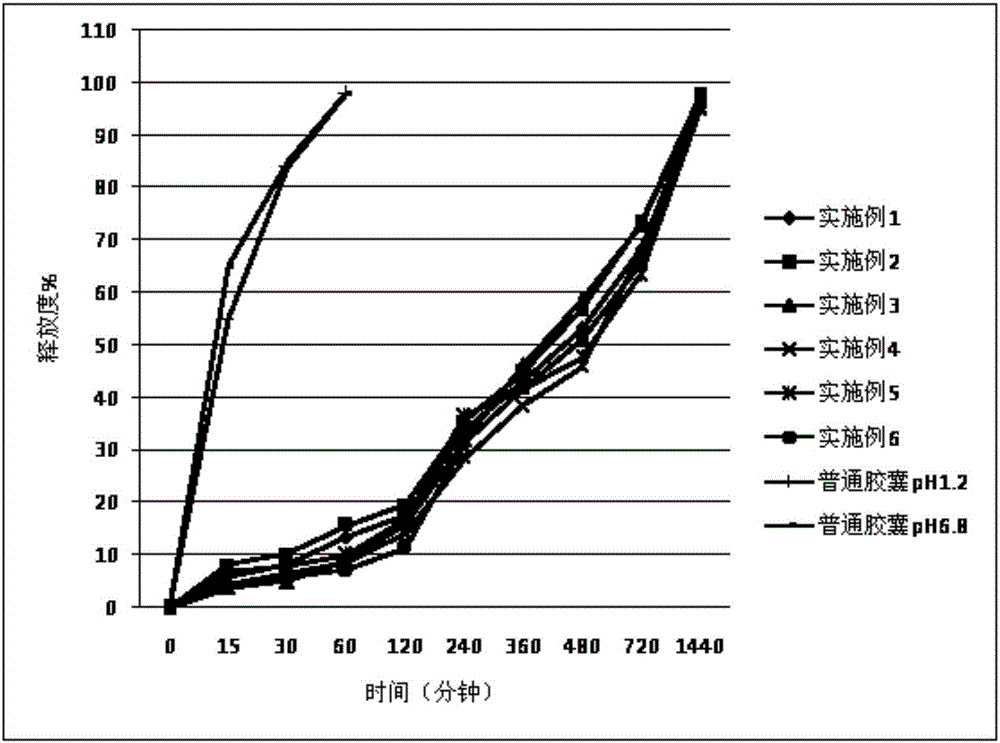
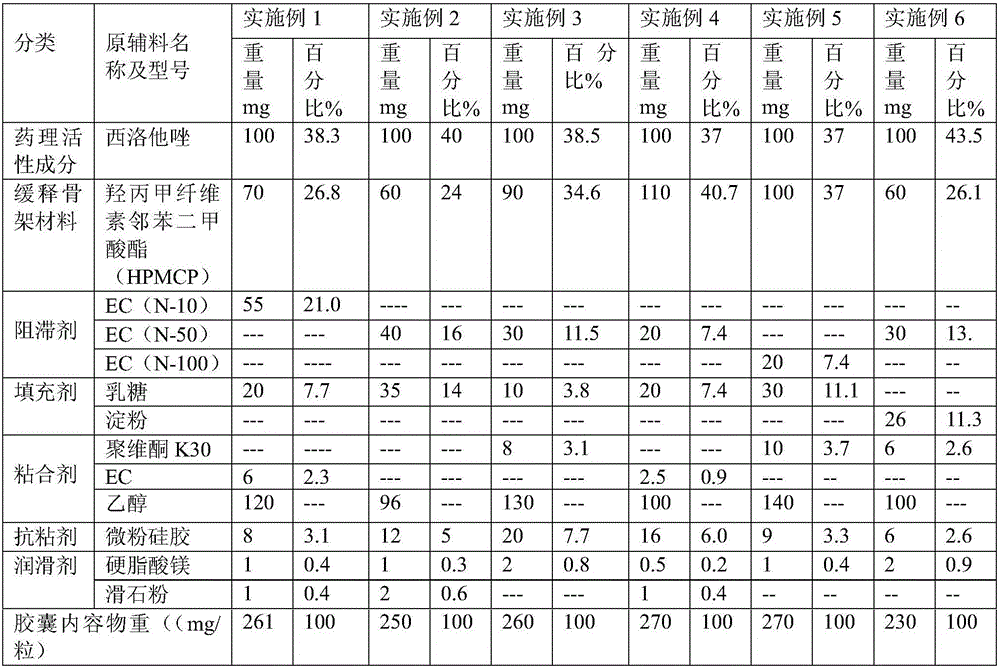
Embodiment 1-6
[0027] The cilostazol sustained-release capsules (specification: 100 mg / capsule) of Examples 1-6 are composed of the raw and auxiliary materials in the following weight ratios as shown in Table 1.
[0028] Table 1
[0029]
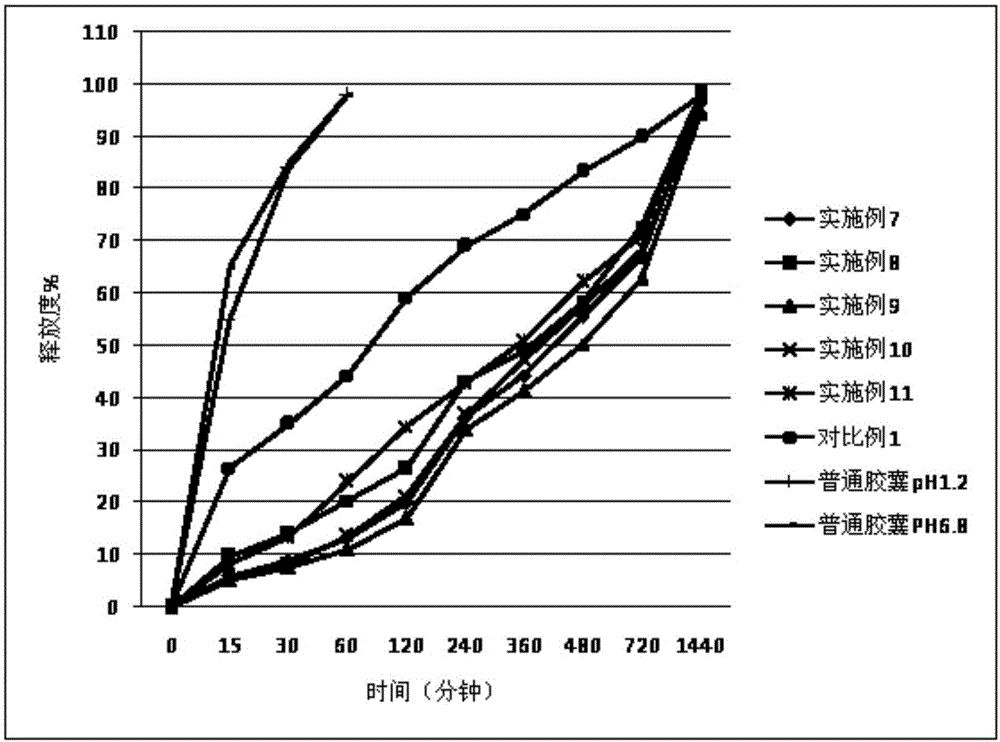
Embodiment 7-11、 comparative example 1
[0031] The cilostazol sustained-release capsules (specification: 200 mg / capsule) of Examples 7-11 and Comparative Example 1 are composed of the following raw and auxiliary materials in the weight ratio as shown in Table 2.
[0032] Table 2
[0033]
[0034]
Embodiment 12
[0036] The preparation method of the cilostazol sustained-release capsules of the present embodiment is as follows:
[0037] The cilostazol is micronized and passed through a 200-mesh sieve before mixing; the anti-sticking agent is passed through a 200-mesh sieve before mixing; the hypromellose phthalate sustained-release skeleton material and blocker Pass through a 80-mesh sieve before mixing; the filler pass through a 100-mesh sieve before mixing; and the lubricant pass through a 100-mesh sieve before mixing.
[0038] Mix the prescribed amount of cilostazol, hypromellose phthalate sustained-release matrix material, blocker, filler, and anti-sticking agent evenly; dissolve the adhesive with ethanol; put the evenly mixed Cilostazol, hypromellose phthalate sustained-release framework material, blocker, filler, anti-adhesive agent made of soft material, granulated twice with 16-24 mesh sieve; After drying at 55°C, sieve with 16-24 mesh, add lubricant and mix well for 15 minutes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com