A method for preparing amides
An amide and ketoxime technology, applied in the field of amide preparation, can solve the problems of equipment corrosion, environmental pollution, low-value ammonium sulfate, etc., and achieve the effects of less by-products, good selectivity and simple treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
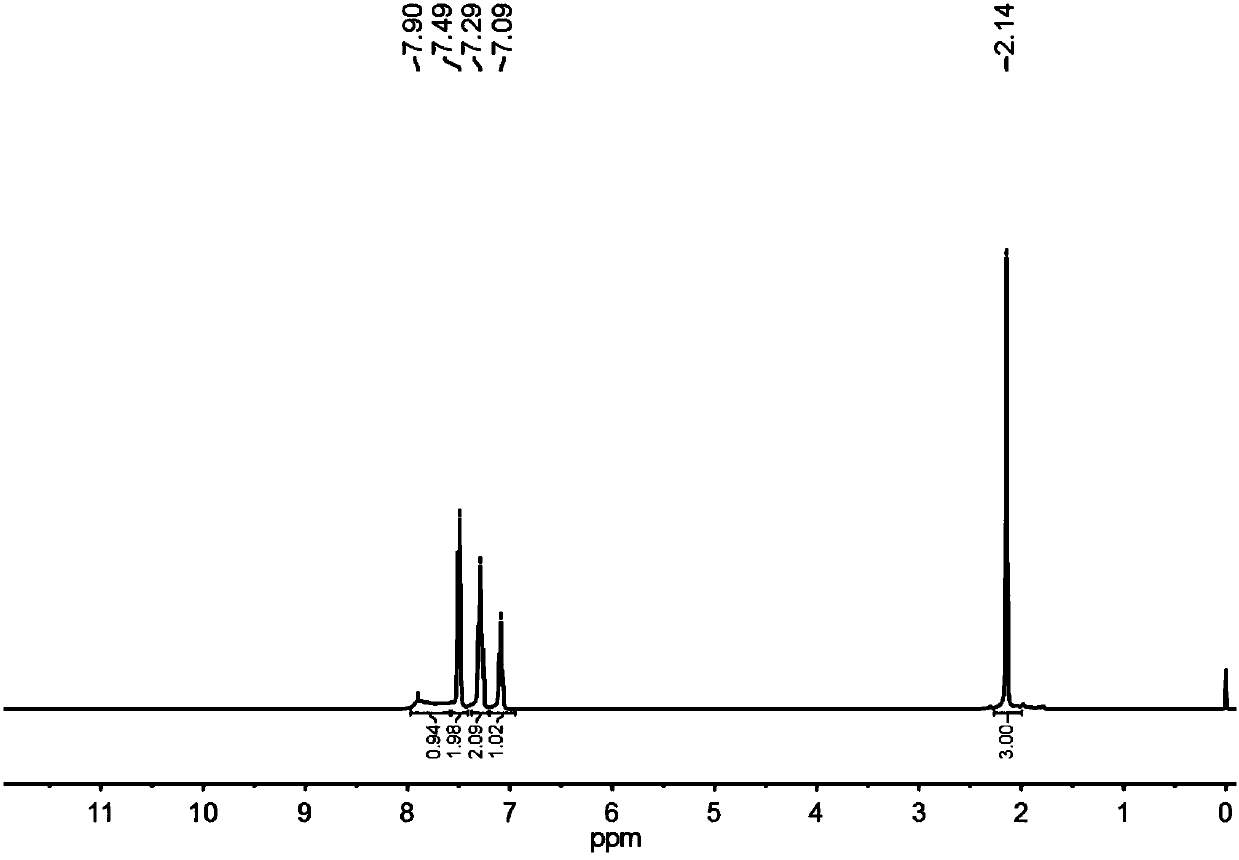
[0029] In a 50ml three-neck round-bottomed flask, add 0.135g of acetophenone oxime, and connect it with a spherical condenser. On the schlenk, perform pumping and ventilation for 3 times. The system is maintained under an Ar atmosphere, then add 10ml of acetonitrile, stir and heat to 80 ℃. Then add tropone and 8.6ul oxalyl chloride of 10ul, reflux reaction, follow up reaction by liquid chromatography analysis (add a little ethyl acetate to dilute in the sample after sampling): 70% of raw material conversion in the time of 10min, raw material is completely in 20min Conversion, the selectivity of target product acetanilide>98%. After the reaction, the solvent was distilled off under reduced pressure, and pure acetanilide was obtained as a white solid by flash preparative chromatography. 1 H NMR (400MHz, CDCl 3 )δ7.90(brs, 1H), 7.49(d, 2H), 7.29(t, 2H), 7.09(t, 1H), 2.14(s, 3H). 13 C NMR (101MHz, CDCl 3 )δ168.90, 138.10, 129.03, 124.39, 120.18, 24.58.
Embodiment 2
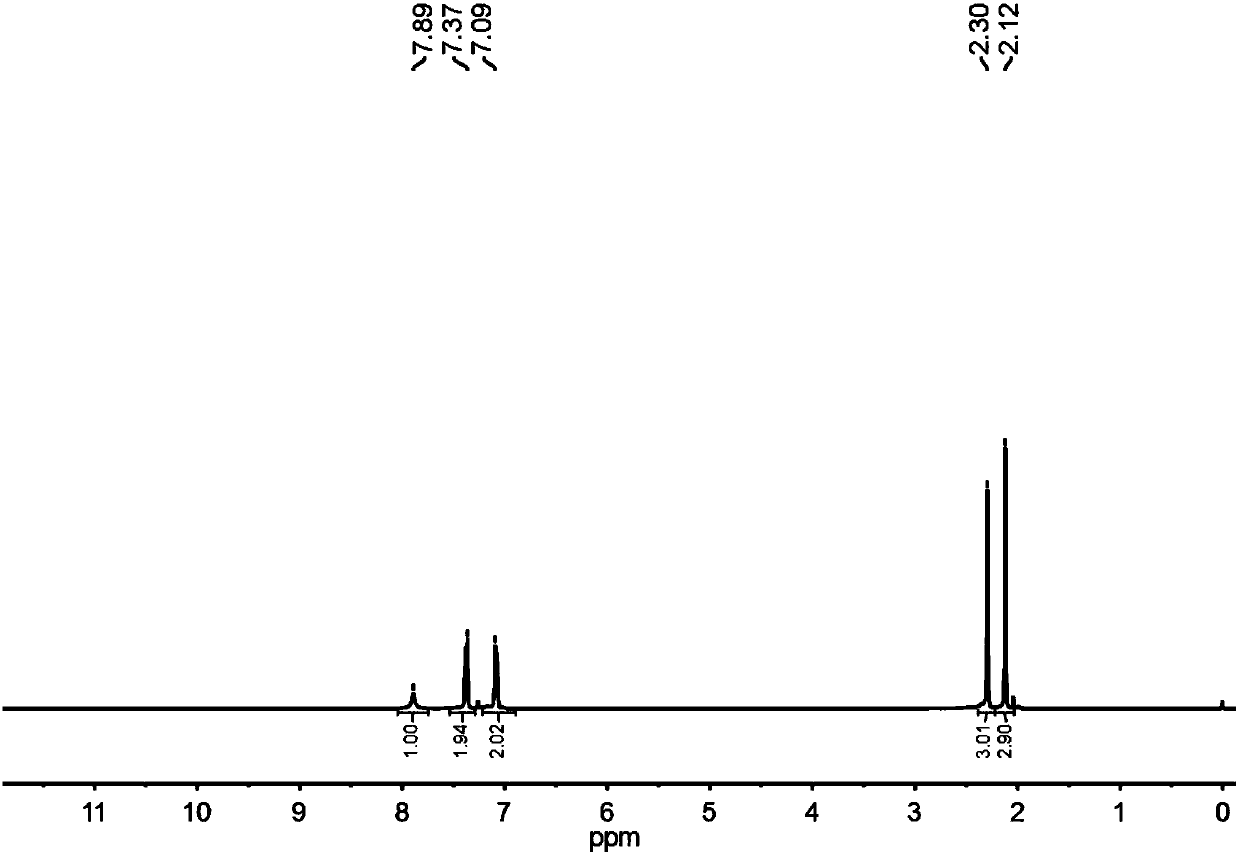
[0031] In a 50ml three-neck round bottom flask, add 0.197g of benzophenone oxime, connected with a spherical condenser, on the schlenk, carry out pumping and ventilation for 3 times, and keep the system under Ar atmosphere, then add 10ml of acetonitrile, stir and heat to 80°C. Then add 10ul tropotrienone and 8.6ul oxalyl chloride, reflux reaction, follow the reaction by liquid chromatography analysis (add a little ethyl acetate to dilute the sample after sampling): 70% of raw material conversion in 30min, complete raw material in 1h Conversion, the selectivity of target product benzanilide>98%. After the reaction, the solvent was distilled off under reduced pressure, and pure benzanilide was obtained as a white solid by flash preparative chromatography. 1 H NMR (400MHz, CDCl 3 )δ7.93(s, 1H), 7.85(t, 2H), 7.64(t, 2H), 7.54(t, 1H), 7.47(t, 2H), 7.36(t, 2H), 7.15(t, 1H ). 13 C NMR (101MHz, CDCl 3 )δ165.93, 138.07, 135.14, 131.94, 129.21, 128.90, 127.16, 124.70, 120.39.
Embodiment 3
[0033] In a 50ml three-neck round bottom flask, add 0.135g of acetophenone oxime and 8mg of 1,1-dichlorocycloheptatriene, and connect it with a spherical condenser. On the schlenk, perform air exchange for 3 times, and the system maintains Under N2 atmosphere, then add 10ml of acetonitrile, stir and heat to reflux reaction at 80°C, follow the reaction by liquid chromatography analysis (add a little ethyl acetate to the sample to dilute after sampling): 72% of raw material conversion in 10 min, complete conversion of raw material within 20 min , the selectivity of target product acetanilide>98%. After the reaction, the solvent was distilled off under reduced pressure, and pure acetanilide was obtained as a white solid by flash preparative chromatography. 1 H NMR (400MHz, CDCl 3 )δ7.90(brs, 1H), 7.49(d, 2H), 7.29(t, 2H), 7.09(t, 1H), 2.14(s, 3H). 13 CNMR (101MHz, CDCl 3 )δ168.90, 138.10, 129.03, 124.39, 120.18, 24.58.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com