LPEIS/DNA/HA nano-carrier and preparation method and application thereof
A nano-carrier and nano-sphere technology, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, gene therapy, etc., can solve problems such as the inability to ensure that genes are not degraded, and there is no targeted immune response, achieving low cost, Controllable size and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
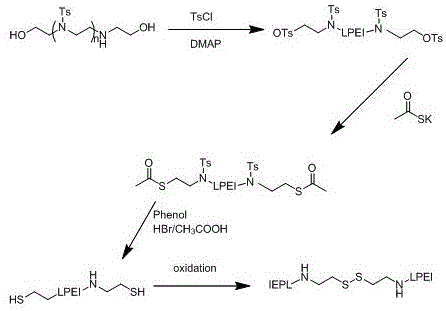
[0057] 1. Sulfonation
[0058] 1.48gN,N-bis(2-hydroxyethyl)ethylenediamine (10mmol) was dissolved in 40ml of dichloromethane, 1.2217g of 4-dimethylaminopyridine (10mmol) was added, 9.9g of trimethylaminopyridine (92mmol ) and 8.3886 g p-toluenesulfonyl chloride (44 mmol). The above mixed solution was stirred at 0°C for 1d. The dichloromethane was evaporated using a rotary evaporator, and the obtained substance was redissolved in 100ml of chloroform, extracted three times with 0.1M hydrochloric acid, and the organic phase was extracted with MgSO 4 Dry and volatilize the organic phase to obtain a white or yellowish powder.
[0059] 2. Desulfonation
[0060] Completely dissolve the powder obtained in 1 in 20ml of N,N-methyleneformamide solution, add excess ethanolamine dropwise, mix and stir the mixture at 40-60°C for 1-2d, and redissolve the obtained substance after volatilizing the organic phase In dimethyl sulfoxide, the organic phase is volatilized to remove residual ethan...
Embodiment 2
[0076] 1. Sulfonation
[0077] 1.48gN,N-bis(2-hydroxyethyl)ethylenediamine (10mmol) was dissolved in 40ml of dichloromethane, 1.2217g of 4-dimethylaminopyridine (10mmol) was added, 9.9g of trimethylaminopyridine (92mmol ) and 8.3886 g p-toluenesulfonyl chloride (44 mmol). The above mixed solution was stirred at 0°C for 1d. The dichloromethane was evaporated using a rotary evaporator, and the obtained substance was redissolved in 100ml of chloroform, extracted three times with 0.1M hydrochloric acid, and the organic phase was extracted with MgSO 4 Dry and volatilize the organic phase to obtain a white or yellowish powder.
[0078] 2. Desulfonation
[0079] Completely dissolve the powder obtained in 1 in 20ml of N,N-methyleneformamide solution, add excess ethanolamine dropwise, mix and stir the mixture at 40-60°C for 1-2d, and redissolve the obtained substance after volatilizing the organic phase In dimethyl sulfoxide, the organic phase is volatilized to remove residual ethan...
Embodiment 3
[0095] 1. Sulfonation
[0096] Dissolve 0.74g N,N-bis(2-hydroxyethyl)ethylenediamine (5mmol) in 20ml of dichloromethane, add 0.6109g of 4-dimethylaminopyridine (5mmol), 4.95g trimethylaminopyridine (46mmol ) and 4.1943 g p-toluenesulfonyl chloride (22 mmol). The above mixed solution was stirred at 0°C for 1d. The dichloromethane was evaporated using a rotary evaporator, and the obtained substance was redissolved in 50ml of chloroform, extracted three times with 0.1M hydrochloric acid, and the organic phase was extracted with MgSO 4 Dry and volatilize the organic phase to obtain a white or yellowish powder.
[0097] 2. Desulfonation
[0098] Completely dissolve the powder obtained in 1 in 20ml of N,N-methyleneformamide solution, add excess ethanolamine dropwise, mix and stir the mixture at 40-60°C for 1-2d, and redissolve the obtained substance after volatilizing the organic phase In dimethyl sulfoxide, the organic phase is volatilized to remove residual ethanolamine to obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com