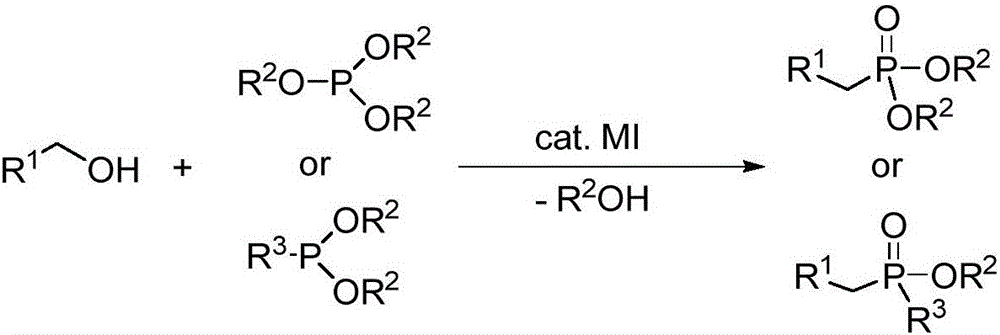
Synthetic method for alkyl group phosphorous acid diester compounds or alkyl group phosphinic acid ester compounds
A technology of alkylphosphonite diester and alkylphosphinate, which is applied in the field of chemical synthesis, can solve the problems of poor stability, many reaction by-products, difficult purification, etc., and meet the requirements of low reaction conditions and environmental protection pollution Effects of small, broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of Diethyl Benzyl Phosphinate from Triethyl Phosphite and Benzyl Alcohol
[0024]
[0025] Benzyl alcohol (54.0mg, 0.50mmol), triethyl phosphite (166.0mg, 1.0mmol, 2.0equiv.) and tetrabutylammonium iodide (3.7mg, 0.01mmol, 2mol% ), vacuumed under nitrogen protection, and then heated to 120°C for 24h under solvent-free conditions. After the completion of the reaction monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 90%. 1 H NMR (500MHz, CDCl 3 )δ7.44–6.87(m,5H),3.99–3.84(m,4H),3.08(d,J=21.5Hz,2H),1.17(t,J=7.0Hz,6H). 13 C NMR (125MHz, CDCl 3 )δ131.62(d, J=9.0Hz), 129.77(d, J=6.5Hz), 128.50(d, J=3.0Hz), 126.84(d, J=3.6Hz), 62.09(d, J=6.8 Hz), 33.79(d, J=138.2Hz), 16.34(d, J=6.0Hz). 31 P NMR (202MHz, CDCl 3 )δ26.48(s).
Embodiment 2
[0027] Preparation of Diethyl 4-Fluorobenzylphosphonite from Triethyl Phosphite and 4-Fluorobenzyl Alcohol
[0028]
[0029] 4-Fluorobenzyl alcohol (63.0mg, 0.50mmol), triethyl phosphite (166.0mg, 1.0mmol, 2.0equiv.) and tetrabutylammonium iodide (3.7mg, 0.01mmol) were sequentially added to a 20mL tubular reactor ,2mol%), vacuumed under nitrogen protection, and then heated to 120°C for 24h under solvent-free conditions. After the reaction was complete as monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 81%. 1 H NMR (500MHz, CDCl 3 )δ7.29–7.24(m,2H),7.00(t,J=8.5Hz,2H),4.07–3.90(m,4H),3.12(d,J=21.5Hz,2H),1.25(t,J =7.0Hz,6H). 13 C NMR (126MHz, CDCl 3 )δ161.93(dd, J=245.3,3.9Hz),131.24(dd,J=7.9,6.7Hz),127.31(dd,J=9.2,3.3Hz),115.41(dd,J=21.5,3.0Hz) ,62.18(d,J=6.8Hz),32.90(d,J=139.2Hz),16.35(d,J=6.0Hz). 31 P NMR (202MHz, CDCl 3 )δ26.15 (d, J=5.7Hz).
Embodiment 3
[0031] Preparation of Diethyl 4-Bromobenzylphosphonite from Triethyl Phosphite and 4-Bromobenzyl Alcohol
[0032]
[0033] 4-Bromobenzyl alcohol (93.0mg, 0.50mmol), triethyl phosphite (166.0mg, 1.0mmol, 2.0equiv.) and tetrabutylammonium iodide (3.7mg, 0.01mmol) were sequentially added to a 20mL tubular reactor ,2mol%), vacuumed under nitrogen protection, and then heated to 120°C for 24h under solvent-free conditions. After the completion of the reaction monitored by TLC, the product was separated and purified by column chromatography, and the separation yield was 90%. 1 H NMR (500MHz, CDCl 3 )δ7.44(d,J=8.0Hz,2H),7.17(dd,J=8.0,2.0Hz,2H),4.10–3.94(m,4H),3.09(d,J=21.5Hz,2H), 1.25(t,J=7.0Hz,6H). 13 C NMR (126MHz, CDCl 3 )δ131.64(d, J=3.0Hz), 131.43(d, J=6.6Hz), 130.78(d, J=9.1Hz), 120.93(d, J=4.6Hz), 62.22(d, J=6.8 Hz), 33.28(d, J=138.7Hz), 16.37(d, J=5.9Hz). 31 P NMR (202MHz, CDCl 3 )δ25.51(s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com