Recombinant human collagen and its coding gene and preparation method thereof
A technology of human collagen and human collagen, which is applied in the field of bioengineering, can solve the problems of difficulty in expressing products, difficulty in product purification, affecting product quality, etc., and achieves the effects of high expression, high purity and good water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0103] The preparation method of the above-mentioned gene recombinant human collagen comprises the following steps:
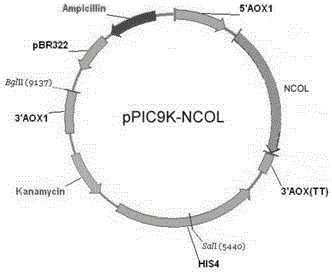
[0104] 1. Construction of gene recombinant human collagen expression vector
[0105] According to the amino acid sequence of the designed gene recombinant human collagen, according to the codon preference of Pichia pastoris, design and artificially synthesize the human collagen nucleotide sequence, and add the XhoI endonuclease cleavage site CTCGAG at the 5' end And Pichia pastoris signal peptide cleavage site sequence AAAAGA, 3' end added EcoRI endonuclease cleavage site GAATTC, the design sequence was synthesized by Shanghai Sangon Bioengineering Co., Ltd., named NCOL, the sequence is as follows, see the sequence list for details SEQ ID No: 3:
[0106]
[0107] XhoI and EcoRI double enzyme digestion was performed on pPIC9K and human collagen synthesized by artificial whole gene, and the digested products were recovered, ligated with DNA ligase, transforme...
Embodiment 1
[0123] 1. Construction of gene recombinant human collagen expression vector
[0124] 1.1 Gene acquisition
[0125] According to the amino acid sequence of the designed gene recombinant human collagen, according to the codon preference of Pichia pastoris, design and artificially synthesize the human collagen nucleotide sequence, and add the XhoI endonuclease cleavage site CTCGAG at the 5' end And Pichia pastoris signal peptide cleavage site sequence AAAAGA, 3' end added EcoRI endonuclease cleavage site GAATTC, the design sequence was synthesized by Shanghai Sangon Bioengineering Co., Ltd., named NCOL, the sequence is shown in the sequence table SEQ ID No: 3:
[0126] 1.2 Vector pPIC9K and NCOL double digestion
[0127] XhoI and EcoRI double enzyme digestion was performed on pPIC9K and human collagen synthesized by artificial whole gene. pPIC9K was purchased from Invitrogen Company. The double enzyme digestion system is as follows:
[0128]
[0129]
[0130] After addi...
Embodiment 2
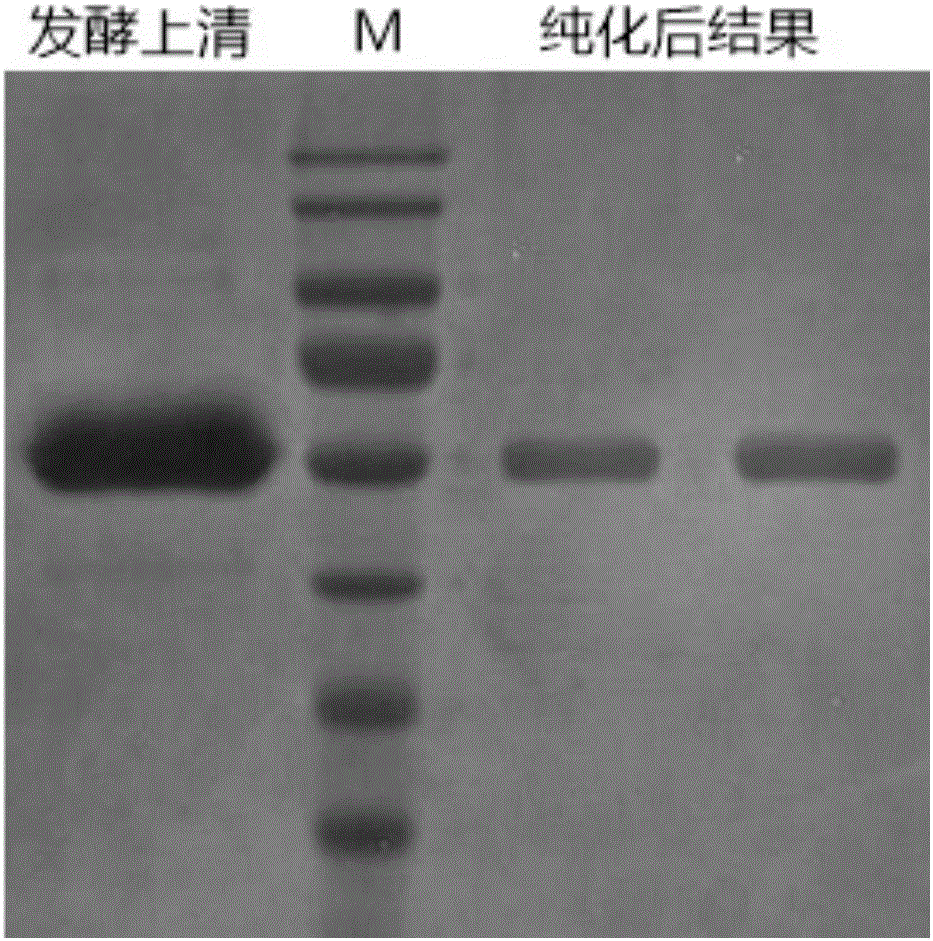
[0155] In step 5 of the purification of genetically recombinant human collagen in Example 1, the hollow fiber microfiltration system with a pore size of 0.1 μm is replaced with a hollow fiber microfiltration system with a pore size of 0.22 μm, and other steps in this step are the same as in Embodiment 1 , the obtained product is also more than 95% genetically recombinant human collagen, its toxicity is 0, and it has good biocompatibility.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com