Determination method of edetate disodium in clevidipine butyrate injection emulsion
A technology of clevidipine butyrate and edetate disodium, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., and can solve problems such as the inability to achieve quality control of EDTA-2Na
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1. Instruments and reagents
[0051] 1.1 Instrument: High performance liquid chromatography is AgiLent 1200Series; chromatographic column is InertsiL ODS-3, purchased from GL Science Inc.; electronic analytical balance is METTLER TOLEDO XS205;
[0052] 1.2 Reagents: Acetonitrile is chromatographically pure, water is ultrapure water, and other reagents are analytically pure.
[0053] 1.3 Drugs: Blank clevidipine butyrate injection emulsion is from Tianjin Kanghong Pharmaceutical Technology Development Co., Ltd.;
[0054] Clevidipine butyrate injection emulsion was purchased from Medicines Co., batch number: 16GF0226;
[0055] Edetate disodium was purchased from USP standard, batch number: JOJ421.
[0056] 2. Chromatographic conditions and system adaptability: octadecylsilane bonded silica gel is used as filler;
[0057] Mobile phase: A phase and B phase composed of organic phase and aqueous phase respectively;
[0058] Wherein: A phase is the tetrabutylammonium hydro...
Embodiment 2
[0098] The control experiment of embodiment 2 sample processing
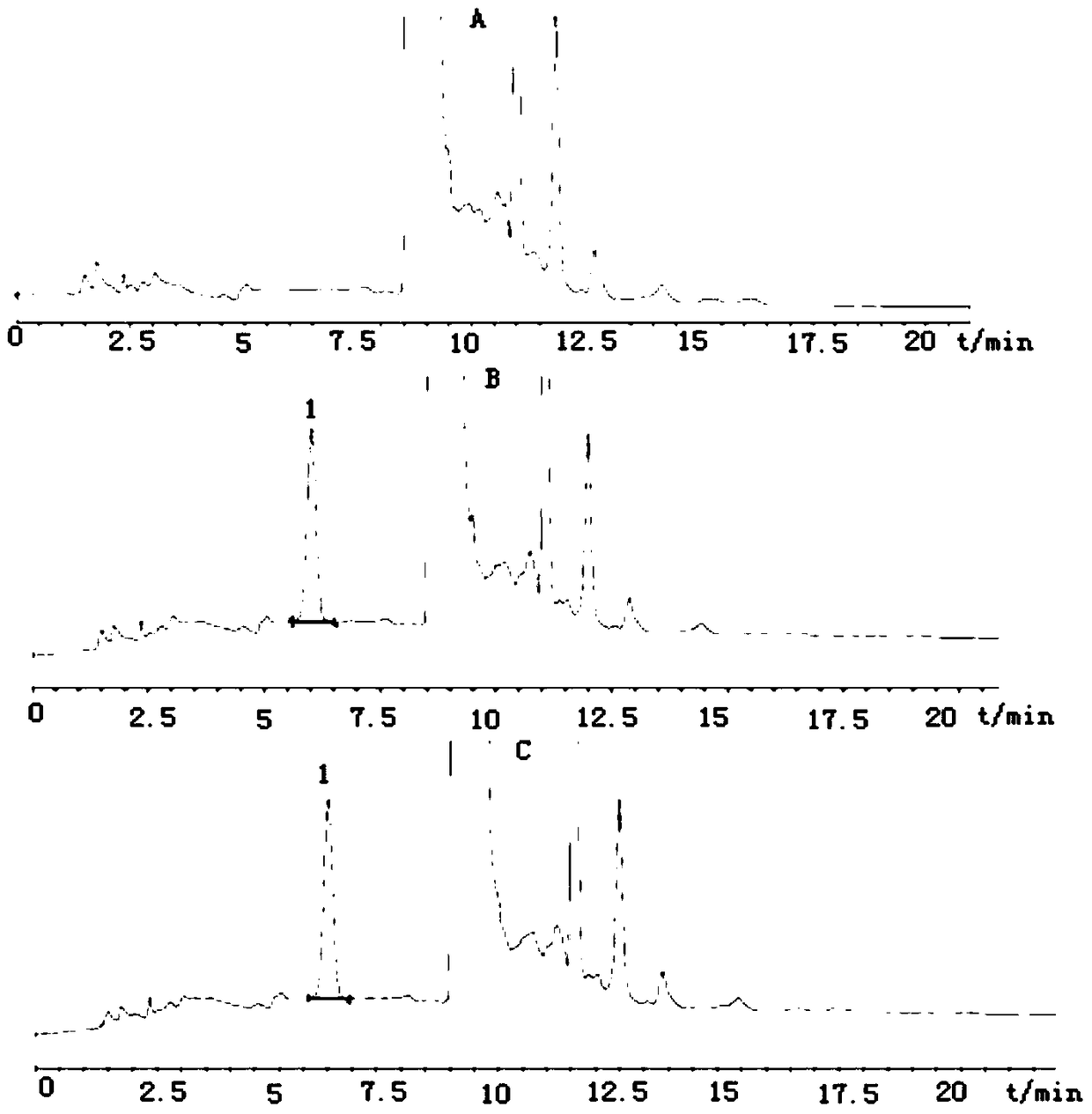
[0099]Three batches of clevidipine butyrate injection emulsion (batch numbers: 1312102131-1, 1312112131, 1312122131) were taken, and the content of edetate disodium was determined according to the following conditions.
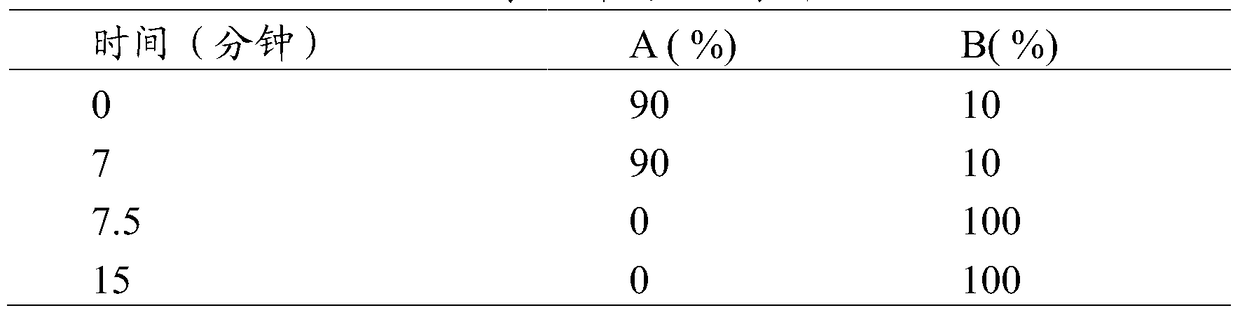
[0100] The chromatographic column adopting octadecylsilane bonded silica gel as filler; mobile phase A phase is 25mmoL / L tetrabutylammonium hydroxide solution (adjust pH6.4 with phosphoric acid)-acetonitrile volume ratio is 4:1, mobile phase Phase B is a methanol-water volume ratio of 9:1, and the gradient elution program is shown in Table 1. The detection wavelength is 254nm, the flow rate is 1.0mL / min, the column temperature is 35°C, and the injection volume is 50μL.
[0101] Sample processing method:
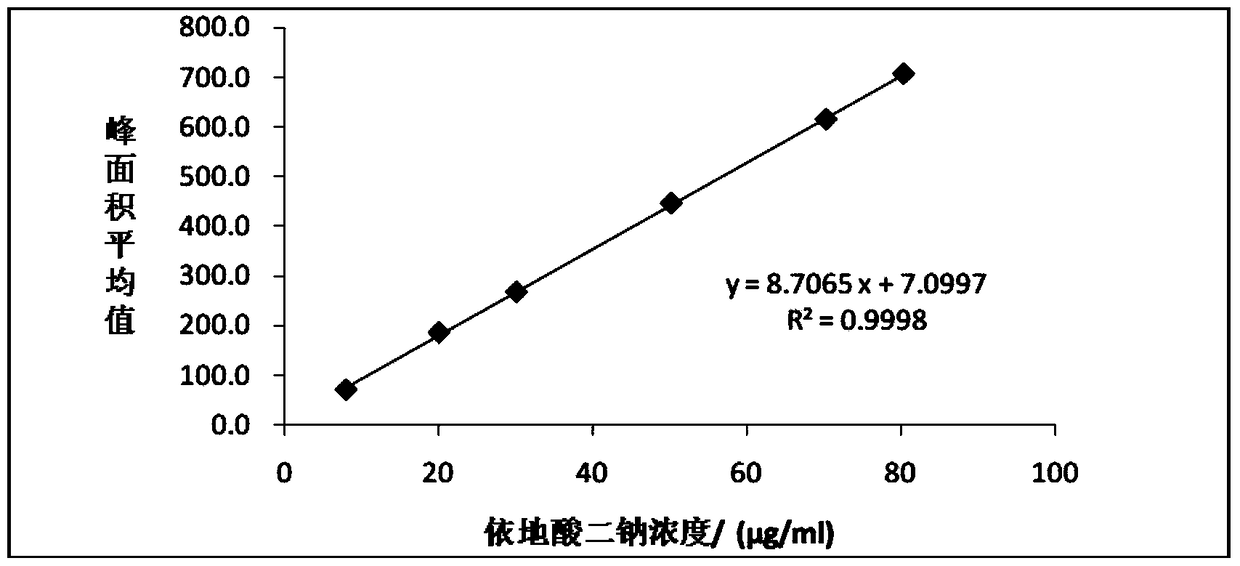
[0102] 1) Precisely measure 1 mL of 500 μg / mL edetate disodium solution, put it in a 50 mL centrifuge tube, add 9 mL of blank clevidipine butyrate injection emulsion, and precisely add isop...
Embodiment 3
[0109] Embodiment 3 chromatographic conditions optimization
[0110] Three batches of clevidipine butyrate injection emulsion (batch numbers: 1312102131-1, 1312112131, 1312122131) were taken, and the content of edetate disodium was determined according to the following conditions.
[0111] Chromatographic conditions: a chromatographic column using octadecylsilane bonded silica gel as a filler; the detection wavelength is 254nm, the flow rate is 1.0mL / min, the column temperature is 35°C, and the injection volume is 50μL.
[0112] (1) Gradient elution: mobile phase A is 25mmoL / L tetrabutylammonium hydroxide solution (adjust pH 6.4 with phosphoric acid)-acetonitrile volume ratio is 4:1, mobile phase B is methanol-water volume ratio is 9:1, the gradient elution program is shown in Table 1.
[0113] (2) Isocratic elution: mobile phase is 25mmoL / L tetrabutylammonium hydroxide solution (adjust pH 6.4 with phosphoric acid)-acetonitrile-isopropanol volume ratio is 6:3:1, isocratic e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com