A preparation method of iron-cerium-based porous catalyst for removing organic sulfur
A technology of organic sulfur and catalyst, which is applied in the preparation of cerium porous materials, iron-cerium-based porous catalysts, and the iron-containing field, can solve the problems that the process method has not been reported, and achieve short preparation time, high sulfur resistance, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Mix iron nitrate and cerium nitrate in a mass ratio of 1:10 to obtain mixed nitrate; mix MIL-100(Fe) and mixed nitrate in a mass ratio of 1:3, add deionized water and stir for 3 hours, to obtain the mixed solution; the above mixed solution was placed in a blast drying oven and dried at 130°C for 8 hours; the dried mixture was placed in a quartz boat and placed in a tube furnace, in an oxygen-enriched atmosphere and 700 The iron-cerium-based porous catalyst was prepared by calcining at ℃ for 2 hours.
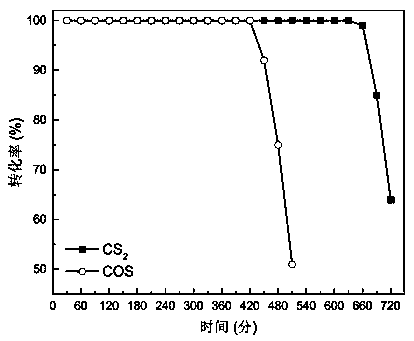
[0028] The activity test of the catalyst was carried out in a fixed-bed quartz reactor, and the reaction conditions were: COS concentration 500ppm, CS 2 Concentration 50ppm, space velocity 50000h -1 , reaction temperature 60℃, oxygen content 5%, COS and CS 2 Catalytic conversion results see figure 1 , it can be drawn from the figure that the conversion rate of 100% COS can be maintained for 420 minutes, and the conversion rate of 100% CS 2 The conversion rate can be ma...
Embodiment 2
[0030] Mix ferric nitrate and cerium nitrate in a mass ratio of 1:5 to obtain mixed nitrate; mix Fe-MOF-5 and mixed nitrate in a mass ratio of 1:0.5, add deionized water and stir for 4 hours , to obtain the mixed solution; the above mixed solution was placed in a blast drying oven and dried at 150°C for 9 hours; the dried mixture was placed in a quartz boat and placed in a tube furnace, in an oxygen-rich atmosphere at The iron-cerium-based porous catalyst was prepared by calcining at lower temperature for 4 hours.
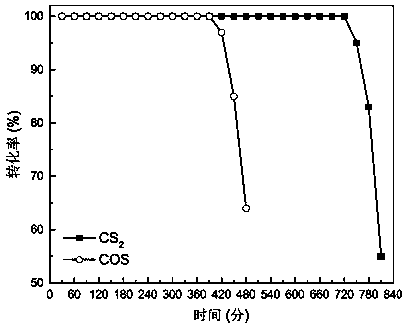
[0031] The activity test of the catalyst was carried out in a fixed-bed quartz reactor, and the reaction conditions were: COS concentration 500ppm, CS 2 Concentration 50ppm, space velocity 50000h -1 , reaction temperature 60℃, oxygen content 5%, COS and CS 2 Catalytic conversion results see figure 2 , it can be drawn from the figure that the conversion rate of 100% COS can be maintained for 390min, and the conversion rate of 100% CS 2 The conversion rate can b...
Embodiment 3
[0033] Mix ferric nitrate and cerium nitrate at a mass ratio of 1:20 to obtain mixed nitrate; mix Fe-MOF-74 and mixed nitrate at a mass ratio of 1:1.5, add deionized water and stir for 5 hours , to obtain the mixed solution; the above mixed solution was placed in a blast drying oven and dried at 100°C for 7 hours; the dried mixture was placed in a quartz boat and placed in a tube furnace, in an oxygen-rich atmosphere at The iron-cerium-based porous catalyst was prepared by calcining at lower temperature for 5 hours.
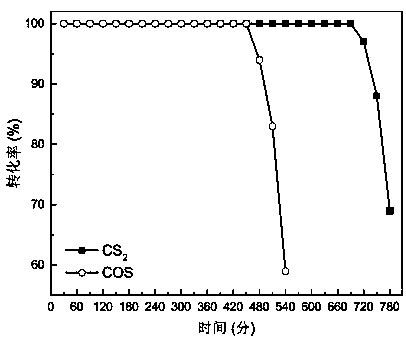
[0034] The activity test of the catalyst was carried out in a fixed-bed quartz reactor, and the reaction conditions were: COS concentration 500ppm, CS 2 Concentration 50ppm, space velocity 50000h -1 , reaction temperature 60℃, oxygen content 5%, COS and CS 2 Catalytic conversion results see image 3 , it can be drawn from the figure that the conversion rate of 100% COS can be maintained for 450min, and the conversion rate of 100% CS 2 The conversion rate can ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com